Tuesday, December 27, 2016 11:15:37 AM
WEBCAST Replay: http://edge.media-server.com/m/p/afyt8u2w (45 Slides)
By: Eb0783 10-13-16 #275973
ASM attendees - roughly 40. 20 of us being shareholders.
FU/275979(re: Garnick?): He was a very valuable asset in the meeting.
FU/276020: From the ASM: King said (in answer to a question about B2GP1 stability, a la GJH) that B2GP1 is “relatively stable” among the patients and, although not as much is known about it compared to other proteins, there probably is no more than a +/-10% variation in a given patient.
Dr Garnick said that B2GP1 is only one (and maybe not the most important) of a number of biomarkers and they will have a number of them creating a profile. Lytle said the $$ spend on Sunrise will be finished by around end of calendar year. My take on these two statements is that they will have the analysis done and the biomarker profile will be complete. imo
Garnick shared a story about an early biomarker at Genentech with us that very few people know. Herceptin failed its first trials but one scientist identified the HER2 mutation and saw how many with HER2 lived longer. So they ran another trial which also failed. Garnick and his peers didn't believe in it but one other guy, identified a biomarker that corrolated with the ones who lived, and talked them into spending another $60mm on one more trial. Since then Herceptin has save 1000's of lives and earned $Billions of dollars. He believes in the value of biomarkers and he has been driving it for us.
As to my question of filing the BLA with what we have, [sorry CP, I did agree with your view) Dr Garnick point blank said our data is “not fileable.” We have now proof of concept and need now another trial, even a small phase II, to validate. Keep in mind however that means a smaller trial and with biomarkers involved it could be shorter than we imagine. imo
On a sidebar with just Shelley and I, Shelly Fussey said they have filed a detailed provisional patent on the Biomarkers which sets the filed date, keep it from being published/shared, and allows inexpensive additions/updates for a number of months (forget if it was 6, 9, or 12). Basically, we the public, and their competitors, will not be able to access it for 18mos. It also adds another year to the patent protection that way.
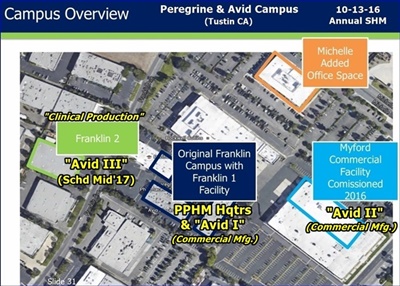
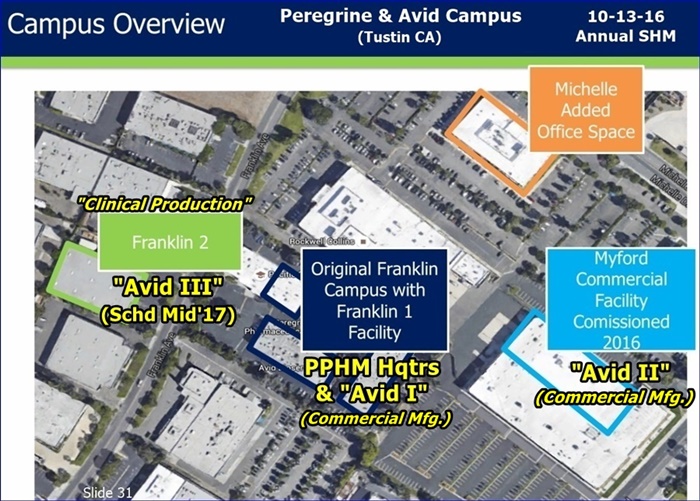
Edit: Avid III has not actually begun construction as the lease is still being negotiated. However the design, equipment models, etc is ready to begin. Some others will probably add some color because they gave 11 of us a tour of the Myford facility. I call that "shareholder friendly."
FU/276047(To HD): King told us before that ps targeting will be an astronomical value and your verbose presence here continues to confirm that for me. Thank you again for your support.
FU/276054(re: Expenses?): You are right. In fact Lytle said they have already reduced expenses by 38% and will be completely finished with Sunrise expense by end of year. Don't believe those who try to say they have not reduced.
FU/276076(Future?): LOL Loofman, You are still here for the same reason the rest of us "believers" are: The potential rewards are Astronomical (even hornet knows it as he is trying to talk us out of our PEARL every day) but it is only taking a little longer.
FU/276334: I wanted to add one more item from the Avid tour. In past Q&A during one of the latest CCs, King talked about how profitable “fill & finish” is and how he wanted to get into that part in the future. Well, part of John Haney’s presentation was a discussion on what “Fill & Finish” is and why we would want to do it (profitable due to difficulties in keeping the product pure). From his explanation, description, and excitement, my take-away is that he has the design, equipment layout, and process already to fit into the next space.
FU/276490(to ExW re: Herceptin data mining analogy): Missing the ASM made you miss this. Herceptin was NOT originally developed for HER2: data mining pointed them there. Garnick said they did a phase III after screening for HER2 and it also failed! Then one more round of "data mining" gave one scientist enough leverage to convince them to do one more. At that time they had nothing else in the pipeline. Sound familiar? With that addl. biomarker screening they ran a successful phase III and the rest is history. That is how I remember it. Some here seem to denigrate and demonize "data mining" but I see the benefit.
FU/276505(to ExW re: Herceptin analogy): I’m just telling you what I think I heard from Dr. Garnick who said, “very few people know this.” That implies to me that it is not included in any public documents. On top of that, one of the most discussed “problems” still today [there is even talk of legislation to correct it] in getting FDA approvals is that BPs often do not include “all” the information (like trial failures) in the BLAs that they file. I’m not making something up, just bringing into question rational reasons why this FDA document may not tell us about failed trials. Why would it? I may be incorrect that it was a phase III as it could have been a phase II but he said they spent $30?mm on the failed trial and this one guy convinced them (even though their committee didn't believe in it) to spend another $60mm on another trial, Phase III. ASM attendees please correct me.
FU/276539(re: Planning future trials): Yes, this is exactly what King said. The biomarker (profile) will let them filter out those who do not benefit and that will be entrance criteria for the next trial which only needs to be about 30% of the Sunrise 600 and that 30% will provide stat.sig. performance (74%?) on OS or other chosen end points. Addl. biomarkers found that can add to the profile will make it even more precise and possibly less than 200 pts could be used.
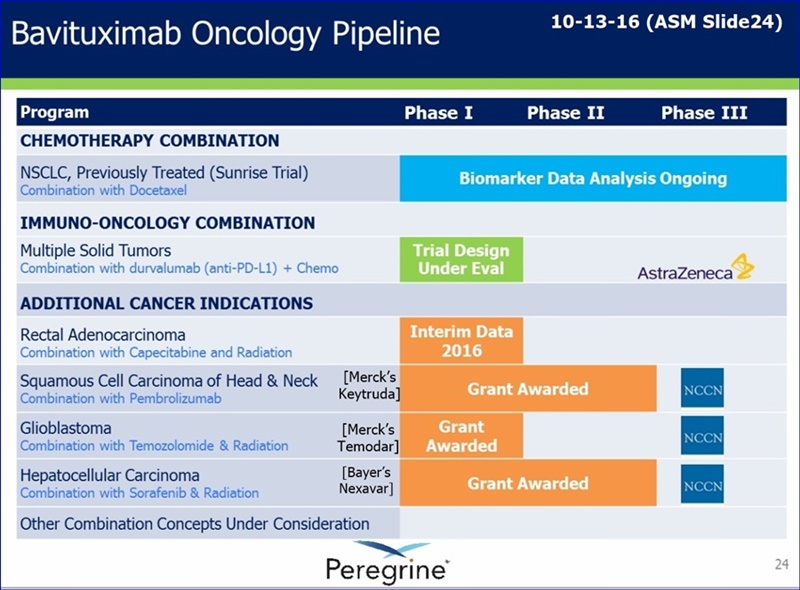
FU/276543: Brandon Cox was talking to Jeff Hutchins & Steve Worsley before the meeting started and much of that was about the aPS + aPD1 + aLAG3 data. I don’t think MSK was mentioned in that discussion (I was listening to some of it). I know he was asking about next steps for that data. I will let Brandon share that with you, if he will.
FU/277081: Jake, At the ASM Q&A King was asked is we were talking with Merck and he succinctly answered, "Yes."
FU/277180(Avid Expansion): Jake, I wish you had been there for the Avid tour so you could hear & see what is happening there. You would have gotten a great deal from it. I spent a number years doing project eng. & mgt. work myself, so I think I can see where some items can be checked off. Peregrine has been a neighbor to the tenants & landlords around them for a few decades. They are well enough known to them, have relationships with them, and have expanded a number of times in that locale over the years. I don’t think the landlords around them have any issues about their credit worthiness. They have surveyed the available sites, have leased some already for various purposes, and they know the advantages/disadvantages of each of them for the purposes they will use them for. The design for the new facility is a smaller but cookie cutter version on Avid II. John Haney also mentioned that the parking used by PPHM at Myford is considerably less than an office tenant would need and he spoke as if that was a plus for them at that location. The only hold-up (time-wise) I see right now is whether they can get the lease signed in the most desirable site or if they will relent and use one that may take a little more infrastructure build-out. It is their timeline and they will decide how much negotiation time is too much (it could be done as we type this). I might also add, imo, that if the demand for a product/service is great enough, there are a myriad of ways to get it financed, including various levels of customer input/$$. I experienced that in another industry.
By: Robert C Jonson 10-13-15 #276028
Eb, I thought I heard Rob Garrick say our trial didn’t give proof of concept, just a correlation with the Bavi + 200-240 group, and another proof of concept trial would have to be done. I asked Steve King about betabodies and he said they're still in the picture and should help out with B2GP1 problem. I also asked about earthquake provisions and he said the production labs have been designed to weather earthquakes, but the ultimate provisions are to expand our mfg. facilities to other locales, possibly to include international sites. I asked him lastly about timing to implement the RS and he said as late as possible to give other upcoming events as much time as possible to raise our pps back to the minimum required (or higher). This ASM was as good as one could be after a company has failed a phase III trial, and the NCCN preliminary data on the triple combination of Bavi, PD-L1, and LAG-3 is particularly exciting. It was particularly fun to socialize with PPHM shareholders, most of whom I knew, but some whom I met for the first time. RCJ
FU/276161: Eb, or North, or someone else who attended the ASM yesterday, please correct or expand me, but in response to a question about advancing our finding of the biomarker, Steve said he'd have to be 99% certain of the correlation first. Brandon asked if that meant a p value of .01 instead of .05 and Steve answered "yes." He then said further analysis of Sunrise data might provide addl. biomarker identification that could bring the confidence level up to 99%.
FU/276173: One person who really impressed me yesterday was John Haney, who I call Peregrine's/Avid's logistician. He conducted the first half of our tour of Avid's facilities and told us that Avid was NOT growing: it is EXPLODING! He said a major reason for this is the tremendous increase in efficiency of the manufacturing process. In the past it was only possible to obtain a few mg's of product from a batch of mix. Now, from the same amount of mix, they are obtaining 2-4 grams of product.
FU/276212: Right, JJ, another surprise for me was that we found out Avid has 10+ customers now vs the 1-2 we had heard previously!
FU/276465: Data mining may not be a preferred means of getting results, but it can be good. Rob Garnick told us at the ASM that without data mining there would be no Herceptin today, a drug that's sold $8-10bb.
FU/276485(to IFU): Rob Garnick brought up Herceptin as an analogy to how/why PPHM is analyzing the Sunrise data.
By North40000 10-14-16 #276066
The attendees at the ASM did not care how timely the meeting started---why should you? Attendees, including us, were quite satisfied talking with management and BOD members pre ASM. The meeting started at 10 or shortly thereafter.
FU/276071: Reverting to an earlier exwannabe post prior to start of ASM, ex is correct no BLA will or would be filed today, per Dr. Garnick's opinion expressed post-close of original ASM presentations...it would be "laughed out of Washington" he said. I rejoined that a lot of funny things happen in Washington these days. Dr. Garnick responded with reliance on his Herceptin experience with Genentech[DNA], and the need--in his opinion--for completion of analysis of Sunrise trial to determine existence of further biomarker data or other data that would provide concrete evidence of validity of the proof of concept data now extant today.
More on this issue when I return to East Coast next week, with access to transcripts or audio record, if any, on what was said.
FU/276075: SK: the RS split will not be immediate, answering your speculation about today or tomorrow. Rather, he said, any RS will be delayed as long as possible. Others who attended likely have already commented to same effect.
FU/276077(Worsley?): Worsely was there. I had no discussions with him, and have no idea what others may have discussed with him before or after ASM.
FU/276086(To Eb0783): We enjoyed visiting with you again, as well as the Avid facilities tour.
FU/276088: As further proxies were collected from attendees at ASM, and were yet to be counted, the actual vote count on proposal 1 was not announced during the meeting itself, only the result. Results themselves were obviously apparent from data and votes on hand during the ASM.
FU/276204: I noted that PPHM had obtained a new stock transfer agent some time ago, and asked whether PPHM had monitored who, or what entity, was buying, selling or accumulating shares of PPHM; I further noted that share price had been cabined in a lid of some sort day to day for some time. PL, CFO, responded to my question and comment. As CP has noted previously on other occasions, and consistent with PL's response, the stock transfer agent is not necessarily aware of certain trades itself known by a certain acronym. As to trades known by the agent, PPHM is aware of them as well as any accumulation > 5%. He reminded us of shareholder protection plan in place that enables certain activities by the BOD. I will not detail those here - they can be posted by others as necessary or desirable.
FU/276323(re: AZN?): The analysis of the Sunrise data in regards to biomarkers is critical to the design of all future trials. Peregrine knows it and so does AZN. AZN is not gone. The future of biomarkers is the ability to zero in on target patients to greatly improve the odds of success while helping patients survive. Any person who attended the ASM would have heard that message from King, Garnick, Shan and others. They have valuable data that could lead to a lighted pathway. According to King, that analysis should be completed by yr-end. The Herceptin story was used as an example by Garnick to illustrate a drug developed from data after failing 2 clinical trials. Garnick also commented that the MOA of Herceptin is still unknown, but as science advances we gain the ability to identify and target specific biomarkers that improve patient outcomes.
FU/276472(re: Avid II & III in Calif): Training purposes for later duplicate facilities to built elsewhere: Dr Garnick's words, not mine.
FU/276598: Dr. Garnick spoke with at least some PPHM ASM attendees, including my wife and me, after the meeting was adjourned: 1) Herceptin experience as he[Dr. Garnick] related it...DNA had only prelim. data that targeting Her2 worked or gave good results in some patients... [DNA] needed further addl. data from another, prospective trial---that trial needed to include the pre-identified Her2 patients. Only a very few, including the original project leader and perhaps Garnick himself, thought THAT addl. trial would produce info that would be useful or that Her2 was in fact useful as a target; top mgt did not. But DNA had nothing more in its pipeline at the time. Finally, or ultimately, top mgt of DNA was convinced to invest what Garnick said was $60mm for that addl. trial. 2) As a further side-note, CJ told me directly that, following the ASM, he and the BOD would convene an extended meeting until what he expected to be early evening hours to discuss various matters/issues....what those might be he did not say, nor did I ask.
FU/276514: "BJ", one of our guides on the tour of the Avid facilities on the afternoon of 10/13/2016, was a neuro-scientist himself with PPHM before switching over to Avid, for how long I did not ask. He originally was from the bay area of SanFran. John Haney, who began our tour, is a mechanical engineer with a great deal of experience in property location & acquisition for purposes intended. Like SK, John had a great many slides shown to us that he employs to demonstrate Avid facilities to customers & visitors. I have not looked on Avid web-site to see what slide decks may be there.
By Djohn 10-13-16 #276058
Expanding head count? Of course. Avid is expanding like wildfire. FDA, biosimilars. 12 customers, $20mm in revenue turned down in 2017 because of capacity constraints.
FU/276225: I would say The GM of biotech in 10 years. From what I saw yesterday PPHM has perfected the manufacturing assemble line of biotech. It will be replicated all over the world IMO. Nice meeting all of you at the ASM and Avid tour!!
By JJ1223 10-14-16 #276197
John Haney was well versed and impressive with his presentation on future Avid growth. I was surprised that Peregrine had to turn down $20MM of business in the past year due to capacity. He made it easy to see Avid's vision of growth and why SK see's total corporate profitability within the next 21mos. Also impressed by his comment of 1-2 new customer visits per month, and their enthusiasm to "get" started. No one I spoke with was happy with the Sunrise results, but the Biomarker data could surprise. We will know by year-end. I am not happy with delay, but I walked away with a clear picture and understanding that my long term investment is probably more secure than at any time in the past. For those who believe Steve King was not positive, I would have to disagree.
FU/276237(to HD): Not true at all. Fact is they tried to lease addl. in the Myford building, but the landlord had made improvements and wanted an unreasonable price. The addl. space in that building remains vacant today. Avid leased across the street at a much better price/sf and a more accommodating landlord. BTW, 6 new clients have already booked for the new facility expected to be operational mid 2017. The search for addl. space is underway at present, with a vision of major expansion planned. "Explosion of business", and "perfect storm" in terms of the business environment were the themes we heard during the Avid tour. Avid now employs 220 people (growing 6 just this week) and the facilities are massive and very impressive. If anyone gets the opportunity to hear John Harney speak about Avid, or tour the facility, you should do so. Sounds like Biosimiliars will play a big role in the future growth of the business at Avid. IMO, Pete Gagnon plays a very important role here.
FU/276487(to IFU re: Herceptin data mining analogy) Perhaps you missed Garnick's point since you did not hear it directly from him. He stressed the importance of analyzing the data by saying that Herceptin failed a PI and PII trial dramatically. No one at Genetech wanted to proceed any further. It was dead until one scientist noticed something in the data and convinced others to take a look. The comparison to Herceptin is not the point. In a Biomarker world, it is critical to understanding.
By Hawkfan1 10-15-16 #276290
I also think that John Haney (Avid Sr.Proj.Mgr, prev: Genentech, Pfizer) is a very impressive gentleman, and his enthusiasm is contagious. It's too bad that he couldn't finish the rest of the Avid tour with us. The tour that Paul and I got with him last year was much better than this year's. I haven't seen anyone else post about this, so I thought I'd add a few details about Avid's current state and future plans. Anyone who was at the ASM, please feel free to make additions or correct any errors. Avid has leased 2 buildings since last year. The one that they are calling the Michelle building (it's a half a block northeast on Michelle Drive) is all office space. They have also leased another building on the other side of Franklin Ave. (I think they called it Franklin II, if I'm not mistaken). They leased this one to be the home of Avid III, or a water purification plant when they couldn't work out a deal with landlord of the Myford building. He had decided to improve his building in the hopes of enticing a Google type business at three times the rate that we are paying to lease Avid II. He added all kinds of amenities (including an outdoor seating area with a fire pit!) to attract creative programmer types, but a warehouse district is not the most desirable location for this type of business, and it has been vacant for the past year. John has been negotiating with the owner, and while he stopped short of saying that he is becoming more reasonable, he noted that he has investors too, and John is hopeful that they can come to some kind of suitable arrangement. He would like to lease the other half of the Myford building to build a water purification plant to support Avid. Currently, Avid buys the water that they use in the cell growing process in barrels. I've forgotten how many gallons John said that they use, but each run costs about $150,000.00 just for the water that they buy. If he is successful in negotiating a lease of the Myford building, then the Franklin II building would be used for warehouse space. The Myford building has a limited amount, but not enough. John also talked a little about some pf the challenges of building the water purification plant. Interestingly, in it's finished state, the water is so pure that it can leach the ions out of the stainless steel pipes that carry it, and they must keep the water circulating to prevent this. Before John had to leave, he also made the comment that he is already looking for his next building, which I assume would be the home of Avid IV, since he has already made other plans for the other 2 buildings.
FU/276506(to ExW re: Herceptin data-mining analogy): My purely uneducated guess as to why we didn't data mine the phII is that there were insufficient numbers in that study to achieve statsig. in any subgroups that they might have found, so it wouldn't have meant much. Just a guess… Regarding the Herceptin trial, I have in my ASM notes that the PhI showed safety, but the phII didn't show much, if any, benefit at all. Most of the powers that be at Genentech (including Garnick) were for dropping the drug, but as eb points out, they had nothing else in the pipeline. Then Dr. Cowen re-analyzed the data, and found that a subgroup of patients that over-expressed Her2 had benefited. He developed a test for Her2 expression, and convinced Genentech to run a $60mm PhIII that pre-selected for the over-expression of Her2, and the rest is history. Hope that clarifies some of the confusion.
By Copper888 10-18-16 #276580
Thanks to all attendees of the ASM for your great recaps and it was a great pleasure to meet you and spend some time together! Everyone did a great job of recapping the discussion portion of the meeting, so I don't have much to add except for a few observations and verifications.
1. B2GP1 discussion - I wrote in my notes several times the phrase "layering of biomarker data" and "layering selection criteria" They have the B2GP1 data but are looking for all the data to come in to further "explain the effects" of the stat sig population. New Data is coming in "every day" and should be complete in the next 3 months. They stated that they want to be 99% sure before moving forward with any confirmatory trials, whether that is a registrational PHII or Phase 3 trial.
2. Avid - John Haney indeed described the business as exploding and attributed the growth to what he called the "perfect storm" of market factors aiding growth including:
i) FDA now has a clear path for approval of biosimilars
ii) Production Tech has now improved to the point that, in his words, "little Avid" can produce a worldwide supply of a given drug and compete with the largest producers of biologics.
He also mentioned that the speed to enter into production agreements used to be a year long courtship. Now companies are begging to get on the schedule and using early test runs just to get a relationship with Avid and hopefully try to jump the line.
They expressed their interest in building new facilities - mentioning places like Europe, Singapore, and other parts of the US taking advantage of local tax incentives to build and to decentralize for risk mitigation (earthquakes and such).
Now comes my personal gut feeling about what I heard...let the chastising begin :)
I think there is a shift on how the company execs and BOD view the business. As mentioned by multiple posters, SK said that they are still looking to hit a "homerun" with Bavi. But I think that they are now doing that in a framework of risk avoidance, and profitability as their primary goals. With every initiative mentioned, SK would talk about partnering in the next sentence. Exosome testing with a partner; potential of exploring the utility of Beta Bodies - would "advance aggressively with a partner"; If Sunrise data warrants a small study to confirm, they would "partner the next step", etc. I think that for good or bad...the new company directive is the march toward profitability. He also said that the company is worth multiples of its current market cap and that they want to delay the RS as much as they can. "I am focused on getting the Share price over a dollar" He mentioned that there may be many events between now and April that may get us there. Well, that is it...I tried to furiously write direct quotes as much as I could. For those in attendance, Please feel free to correct or add to anything written here… Hope that this is of some value!
WEBCAST Replay (10-13-16 ASM): http://edge.media-server.com/m/p/afyt8u2w (45 Slides)
Slideshow PDF (45 Slides): http://files.shareholder.com/downloads/PPHM/2879585795x0x911689/C8068896-AF8B-4CAA-A08C-FB483775B2AD/20161013-_PPHM_Corporate_Overview_-_ASM_FINAL.pdf
EXCERPTS:

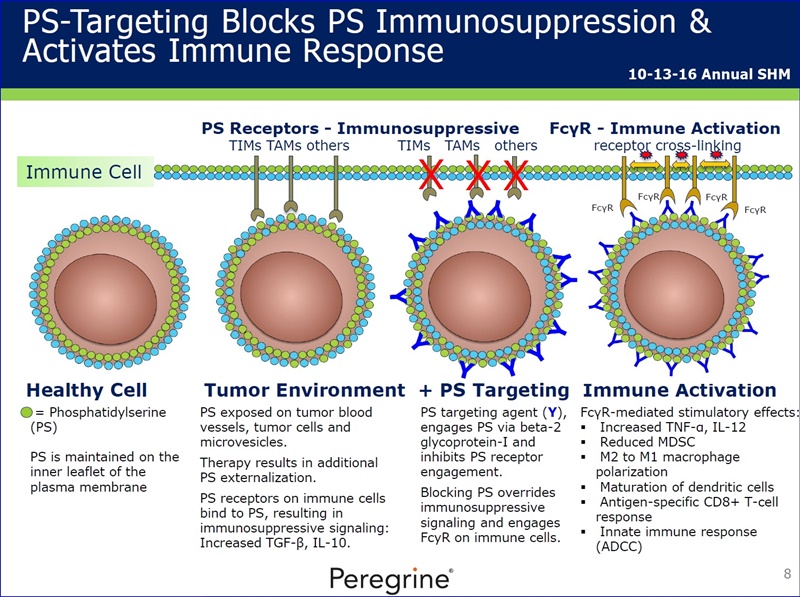


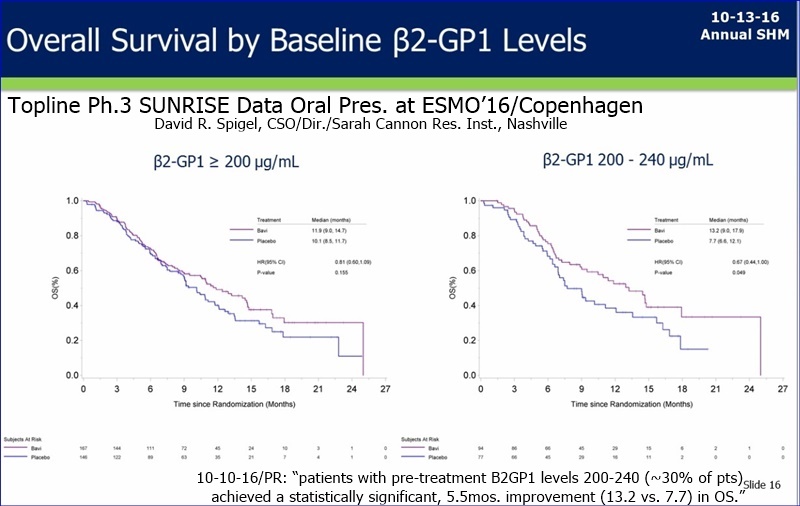
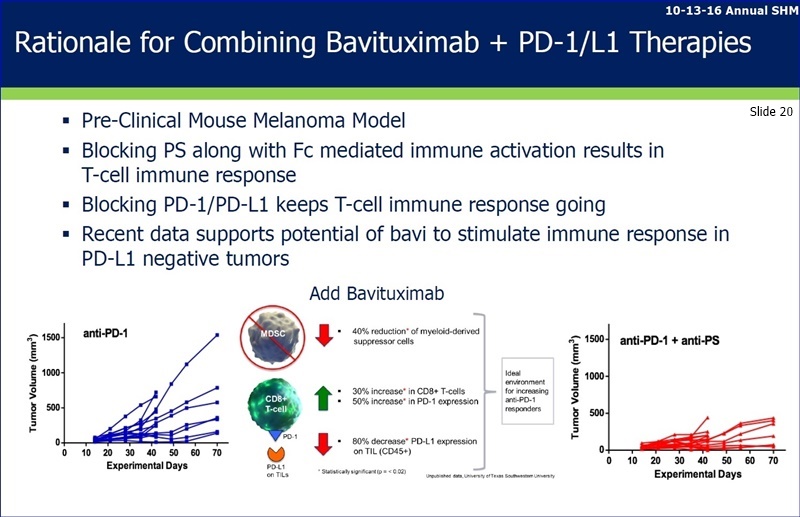


----------
BAVI MOA 10-24-16: Duke’s Herbert K. Lyerly (w/PPHM) poster on AntiPS/TNBC data at AACR’s Tumor Immunotherapy Conf./Boston http://tinyurl.com/zzryfok
...”Modulating The Tumor Microenvironment to Enhance Cancer Immunotherapy by Inducing Phosphatidylserine Expression on the Tumor Surface”
Nov9-13 2016: (SITC’16) Society for Immunotherapy of Cancer 31st Annual Meeting, Natl-Harbor MD http://tinyurl.com/j4tw5p9
...”First Results” from our collaboration with Jedd Wolchok Lab investigators (MSK) to be presented” (per 9-8-16/Ccall/Hutchins)
...I. “PS Targeting Antibody in Combo w/Checkpoint Blockade & Tumor Rad. Therapy Promotes Anti-Cancer Activity in Mouse Melanoma” - Sadna Budhu, PhD - Ludwig Collaborative Lab, MSKCC
...II. “Antibody Targeting of PS Enhances the Anti-Tumor Responses of Ibrutinib & anti-Pd-1 Therapy in a Mouse Triple Neg. Breast Tumor Model” - Jian Gong, PhD, PPHM
...III. Monoclonal Antibodies Targeting PS Enhance Combinational Activity of the the Immune Checkpoint Targeting Agents LAG3 & PD-1 in Murine Breast Tumors” - Michael Gray, PhD, PPHM
...Dr. Jedd Wolchok spoke about Bavi in the 11-14-16/PR (SITC’16, Joint MSKCC & PPHM Poster, “Phosphatidylserine Targeting Antibody in Combination with Checkpoint Blockade & Tumor Radiation Therapy Promotes Anti-Cancer Activity in Mouse Melanoma”)…
11-14-16: "Based on these study results, we believe that the targeting of PS is having meaningful activity within the tumor microenvironment in the B16 melanoma model," stated Dr. Jedd Wolchok. "It appears that this activity creates a more immune active environment in which other treatments, including radiation, are able to have a greater anti-tumor impact." http://tinyurl.com/js3fca4
11-14-16: "We have noted that the combination of PS-targeting treatment and radiation, as well as triple combination of PS-targeting treatment, radiation and anti-PD-1, resulted in clear advantages in anti-tumor activity in the mouse B16 melanoma model," said Dr. Taha Merghoub, PhD, co-director of the Ludwig Collaborative Laboratory at MSK. "We believe that these findings suggest the potential benefit of combining these agents to improve the outcomes of patients with cancer. With this in mind, we think this research may play an important role in designing future clinical trials of PS-targeting agents in melanoma and other cancers." http://tinyurl.com/js3fca4
Note: FULL SITC’16 ABSTRACT #199: http://bit.ly/2dHTEVn
CONCLUSION SECTION FROM THE JOINT MSKCC/PPHM SITC’16 POSTER:


Recent CDMO News
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 07/16/2024 11:50:20 PM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 07/16/2024 11:48:19 PM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 07/15/2024 08:40:04 PM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 07/12/2024 08:30:04 PM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 07/11/2024 09:02:39 PM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 07/11/2024 09:02:27 PM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 07/11/2024 09:01:22 PM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 07/11/2024 09:01:05 PM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 07/11/2024 09:00:54 PM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 07/11/2024 09:00:45 PM
- Form 144 - Report of proposed sale of securities • Edgar (US Regulatory) • 07/11/2024 12:23:26 AM
- Avid Bioservices Reports Financial Results for Fourth Quarter and Fiscal Year Ended April 30, 2024 • GlobeNewswire Inc. • 07/02/2024 08:05:04 PM
- Avid Bioservices to Report Financial Results for Quarter and Fiscal Year Ended April 30, 2024, After Market Close on July 2, 2024 • GlobeNewswire Inc. • 07/01/2024 11:00:21 AM
- Avid Bioservices Earns Committed Badge from EcoVadis for Sustainability Performance • GlobeNewswire Inc. • 05/23/2024 12:05:46 PM
- Avid Bioservices to Participate at Upcoming Investor Conferences • GlobeNewswire Inc. • 05/07/2024 08:05:11 PM
- Avid Bioservices Reports Financial Results for Third Quarter Ended January 31, 2024 • GlobeNewswire Inc. • 04/24/2024 09:25:33 PM
- Avid Bioservices Announces Receipt of Deficiency Notice from Nasdaq Regarding Late Form 10-Q • GlobeNewswire Inc. • 03/20/2024 11:00:10 AM
- Form 8-K - Current report • Edgar (US Regulatory) • 03/07/2024 11:30:11 AM
- Avid Bioservices Announces Pricing of Private Placement of Convertible Notes • GlobeNewswire Inc. • 03/07/2024 04:58:48 AM
- Avid Bioservices Announces Proposed Private Placement of Convertible Notes • GlobeNewswire Inc. • 03/06/2024 09:32:07 PM
- Avid Bioservices Announces Certain Preliminary Financial Results for Third Quarter Ended January 31, 2024 • GlobeNewswire Inc. • 03/06/2024 09:31:28 PM
- Form 8-K - Current report • Edgar (US Regulatory) • 03/06/2024 09:30:18 PM
- Form SC 13G/A - Statement of acquisition of beneficial ownership by individuals: [Amend] • Edgar (US Regulatory) • 01/26/2024 09:57:52 PM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 01/13/2024 12:34:35 AM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 01/12/2024 12:39:18 AM
ELEMENT79 ANNOUNCES UPLISTING TO OTCQB VENTURE MARKET • ELMGF • Aug 26, 2024 10:03 AM
North Bay Resources Announces Gold Assays up to 2.2 Ounces per Ton, Fran Gold Project, British Columbia • NBRI • Aug 26, 2024 10:00 AM
PickleJar Unveils Latest Venue Managed Services Innovations in Upcoming Webinar • PKLE • Aug 23, 2024 1:11 PM
Element79 Gold Corp Provides Update on Nevada Portfolio • ELMGF • Aug 23, 2024 8:00 AM
Maybacks Adds Award Winning Show to Its Lineup Discusses Maybacks Opportunity • AHRO • Aug 22, 2024 11:30 AM
North Bay Resources Announces First Gold Concentrate at Mt. Vernon Gold Mine, Assays 12 oz/ton Gold, 17.5 oz/ton Platinum, and 8 oz./ton Silver, Sierra County, California • NBRI • Aug 22, 2024 10:28 AM







