| Followers | 449 |
| Posts | 41478 |
| Boards Moderated | 5 |
| Alias Born | 09/26/2009 |
Friday, April 29, 2016 9:24:45 AM
#IoT #WearableTechnology #Vision $JNJ $EPGL #SmartContactLens
Johnson & Johnson Vision Care, Inc. Secures Exclusive Right of First Negotiation to EP Global Communications, Inc. Ophthalmic Technologies -> Filed Via OTC Markets
http://www.bizjournals.com/prnewswire/press_releases/2016/04/25/CG79943
As a disclosure to shareholders, on April 12, 2016, Johnson & Johnson Vision Care, Inc. has secured an exclusive right of first negotiation to EP Global Communications, Inc. ophthalmic technologies via written Agreement between the parties.

"We can confirm that the picture is our lens and our technology which has been proven to work while keeping perfect clarity in the Silicone Hydrogel Lens." -EPGL
"The EPGL technology pictured was manufactred, tested and proven in Coopervision lenses at Coopervision. Coopervision now has license to it, but EPGL owns it." - EPGL
***MUST LISTEN***->>EPGL Conference Call 4/28/16
$EPGL "MAJOR PLAYER" in $4,000 detailed Pipeline Assessment Report ->Contact Lenses Market Medical Devices Pipeline Assessment 2016 Research Report
http://www.prnewswire.co.in/news-releases/contact-lenses-market-medical-devices-pipeline-assessment-2016-research-report-577070361.html
RnRMarketResearch.com adds "Contact Lenses - Medical Devices Pipeline Assessment, 2016" market research report to its database, which provides comprehensive information on the pipeline products with comparative analysis of the products at various stages of development. The report reviews major players involved in the pipeline product development. It also provides information about clinical trials in progress, which includes trial phase, trial status, trial start and end dates, and, the number of trials for the key Contact Lenses pipeline products.
Contact Lenses Companies and Product Overview discussed in this research are Aeon Astron Corporation, Alcon, Inc., Apeliotus Ophthalmics, Auburn University, Axcelon Biopolymers Corp., Bausch & Lomb Incorporated, Body Organ Biomedical Corp., Children's Hospital Boston, CooperVision, Inc., EP Global Communications, Inc., Euclid Systems Corporation, Eyenovations, EyeYon Medical, Imperial College London, Johnson & Johnson Vision Care, Inc., Massachusetts Eye and Ear Infirmary, Novartis AG, Ocular Dynamics, LLC, Ocutec Limited, Presbia Plc, Stanford University, The Hong Kong University of Science and Technology, The University of New South Wales, UltraVision CLPL, University of California, San Diego, University of Florida, University of Valencia, University of Washington, University of Western Ontario, Valeant Pharmaceuticals International, Inc., Visioneering Technologies, Inc., VISTAKON Pharmaceuticals, LLC and Yolia Health.
Complete report on Contact Lenses Medical Devices Pipeline Assessment with 181 market data tables and 6 figures, spread across 172 pages is available at http://www.rnrmarketresearch.com/contact-lenses-medical-devices-pipeline-assessment-2016-market-report.html .
Scope of this report has extensive coverage of the Contact Lenses under development, details of major pipeline products which includes, product description, licensing and collaboration details and other developmental activities. The report reviews the major players involved in the development of Contact Lenses and list all their pipeline projects. The coverage of pipeline products based on various stages of development ranging from Early Development to Approved / Issued stage. The report provides key clinical trial data of ongoing trials specific to pipeline products as well as recent developments in the segment / industry.
EPGL PUBLISHED PATENT APPLICATIONS!!!!
MEDICAL DEVICE WITH PRE-DEFINED SPACE AND RELATED METHODS
Publication Date: 3/31/2016 Filing Date: 9/22/2015
Application Number:
PCT/US2015/051321
Publication number:
WO2016/048941
Inventor(s):
MARKUS, David T.; c/o EP Global Communications, Inc.
HAYES, Michael C.; c/o EP Global Communications, Inc.
BACK, Arthur; c/o CooperVision, Inc.
TRAN, Victoria; c/o CooperVision, Inc.
MARULLO, Rachel; c/o CooperVision, Inc.
https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2016048941&recNum=1&office=&queryString=PA%3A%28EP+GLOBAL+COMMUNICATIONS%2C+INC%29+&prevFilter=&sortOption=Pub+Date+Desc&maxRec=2
ELECTRONIC MEDICAL DEVICES AND METHODS
Publication Date: 02/11/2016
International Filing Date: 08/05/2015
Publication number:
WO2016/022665
Application Number:
PCT/US2015/043783
Inventor(s):
MARKUS, David T.; c/o EP Global Communications, INC.
HAYES, Michael C.; c/o EP Global Communications, INC.
BACK, Arthur; c/o Coopervision, INC.
https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2016022665&recNum=1&maxRec=1330&office=&prevFilter=&sortOption=Date+de+pub.+antichronologique&queryString=FP%3A%28markus+david%29+&tab=PCT+Biblio
PIEZOELECTRIC ENERGY HARVESTING CONTACT LENS
Pub. No.:WO/2016/014118
International Application No.: PCT/US2015/015516
Filed 02/11/2015
Published 01/28/2016
Inventors:
MARKUS, David T.; c/o EP Global Communications, INC.
HAYES, Michael C.; c/o EP Global Communications, INC.
https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2016014118&recNum=1&maxRec=&office=&prevFilter=&sortOption=&queryString=&tab=PCT+Biblio
ELASTIC CIRCUIT United States Patent Application 20160014889
Filed: 07/08/2015
Published 01/14/2016
Inventors:
MARKUS, David T.; c/o EP Global Communications, INC.
HAYES, Michael C.; c/o EP Global Communications, INC.
https://patentscope.wipo.int/search/en/detail.jsf?docId=US154143847&recNum=6&maxRec=108&office=&prevFilter=&sortOption=Pub+Date+Desc&queryString=FP%3A%28David+T.+Markus%29&tab=NationalBiblio
SYSTEM AND METHOD FOR CONTACT LENS WIRELESS COMMUNICATION
Application Number 61970335
Filed 03/25/2014
Published 09/01/2015
Inventors:
MARKUS, David T.; c/o EP Global Communications, INC.
HAYES, Michael C.; c/o EP Global Communications, INC.
http://appft.uspto.gov/netacgi/nph-Parser?Sect1=PTO2&Sect2=HITOFF&p=1&u=%2Fnetahtml%2FPTO%2Fsearch-bool.html&r=1&f=G&l=50&co1=AND&d=PG01&s1=20150281411&OS=20150281411&RS=20150281411
PIEZOELECTRIC SENSOR FOR VISION CORRECTION
Filed 02/13/2015
Publication 08/20/2015
Pub. No.: WO/2015/123616
International Application No: PCT/US2015/015978
Inventors:
MARKUS, David T.; c/o EP Global Communications, INC.
HAYES, Michael C.; c/o EP Global Communications, INC.
https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2015123616&recNum=1&maxRec=&office=&prevFilter=&sortOption=&queryString=&tab=PCT+Biblio
It seems it takes 26 months in average to have the patent approved.
So make your bet but it is just an average , can be more months.
http://www.uspto.gov/corda/dashboards/patents/main.dashxml?CTNAVID=1004
SYSTEM AND METHOD FOR CONTACT LENS WIRELESS COMMUNICATION
Filing date Mar 25, 2014 + 26 months = May 25 2016
ELASTIC CIRCUIT
Filing date Jul 8, 2014 + 26 months = September 8 2016
PIEZOELECTRIC SENSOR FOR VISION CORRECTION
Filing date Feb 13, 2014 + 26 months = April 13 2016
MEDICAL DEVICE WITH PRE-DEFINED SPACE AND RELATED METHODS
Filing date 23.09.2014 + 26 months = November 23 2016
ELECTRONIC MEDICAL DEVICES AND METHODS
Filing date Aug 05 2014 + 26 months = Oct 05 2016
PIEZOELECTRIC ENERGY HARVESTING CONTACT LENS
Filing date July 21 2014 + 26 months = Sept 21 2016
EPGL said it best:
According to real experts (IP lawyers) it is expected that patents pending go through a process of claims review for "novelty" and "inventive step". It is not uncommon for the largest corporations such as Apple and Google to have multiple claims challenged on the way to ultimate approval by USPTO and International review bodies. The process involves give and take between IP counsel and examiners. EPGL has several non-provisional and PCT applications pending at present. This includes those jointly filed with CooperVision As an example, some existing patents may include similarities in art, but not all claims are exactly alike. It is the IP lawyers who work with the USPTO and PCT to illuminate the important differences.
@EPGLMed $EPGL Energy Harvester in a contact lens gets Novelty and Inventive step on on specific device (claim 5) design. Great start. Just the beginning. We have novelty on EVERY claim on another key patent pending (yet to be made public) - another great start. More coming.
RECENT TWITTER UPDATES ~~~EPIC~~~~~
EPGLMed: To potential EPGL Investors who have not taken a position yet, Here's the bet you are making by investing in EPGL: In 2013 and and 2014, tiny EPGL and Large CooperVision, Inc. teamed up in a Development Agreement. As EPGL team began to prove to in CooperVision R&D head, Dr. Arthur Back, that our innovations actually worked in real, modern, mass produceable, Silicone Hydrogel lenses, CooperVision then signed EPGL to a Second Development Agreement that was bigger and more comprehensive of all EPGL technologies. Joint IP, as a result of the proven concepts, then began to be filed.
Those jointly developed patents originated from experimentation in real-world CooperVision, Inc. manufacturing facilities. We have strong reason to believe that neither Google nor Alcon/Novartis was ahead of EPGL/CooperVision. Nothing is promised, but that is what our best knowledge is.
The potential market for Autofocus enabled contact lenses alone is $5B + annual. The market for other combined vision enhancements and potential AR applications is estimated by top CEO's in technology to be worth $100B.
So, as a potential investor, you are making the bet that tiny EPGL who (through settlement negotiations ) has now gained 100% ownership and control, while allowing CooperVision to be the FIRST to have license, has pending patents that in some cases could "run the table" of this massive potential market if granted.
That is the bet YOU are making if you go long-EPGL, if this tiny OTC is on to something very big as we believe we are. And don't let others try to tell you this situation is too good to be true, because what has just been laid -out for you is the facts, backed by independent documents
So, you decide, after doing due diligence and reading all materials. EPGL is only at 3 cents currently due to low volume moves. The volatility works both ways however. EPGL has a chart history of big gains over time and rising back to pass previous highs. In summary, with our NEW Ownership of the pending patents and WORLDWIDE rights, EPGL is better positioned than ever for growth from IP licensing and partnerships if ONLY ONE of the jointly developed patents we have filed and is pending presently, gets approval, those who use it will have to pay EPGL royalties, whether or not we have a formal license Agreement with them.
The bet? The bet is on IP that may pay-off big if granted and if the coming Vision Revolution is as big as predicted. Literally all things now on your smartphone, are being predicted to be and if the coming Vision Revolution is as big as predicted made available in your field of vision. Think that is big? Good luck with whatever bet you make on EPGL!
Michael Hayes & Dr. Markus at Intel HQ for meeting in 2014 .

Michael Hayes, Dr. David Markus & Dr. Arthur Back at CooperVision, Inc. "Inventing the future of vision."

Michael Hayes and Gavin Rossdale about to watch Federer and Djokavic play in CA.

Michael Hayes and Dr. David Markus in San Francisco.

EPGL and CooperVision, Inc. are not "done" at all. The parties must continue to adhere to their latest Agreement which governs rights and ownership of the jointly developed technologies. This arrangement will go on for years. EPGL intends for CooperVision to be only one of many companies to have license to our newfound ownership and control of the technology. In fact, EPGL is now in process of implementing new plans that will begin to become visible within the next 14 days. Our last PR was misunderstood by many. EPGLs position and potential is now stronger than ever before with what we have gained. 2016 is truly the new beginning. NEW PATENTS PENDING. NEW OWNERSHIP & CONTROL. NEW WORLDWIDE MARKETING RIGHTS. 3 reasons why EPGL is a player in the coming VISION REVOLUTION!
https://twitter.com/EPGLMed
EVIDENCE OF JOINT PATENT FILING WITH MULTI-BILLION DOLLAR NYSE LEADER.
Kilyck & Bowersox, P.L.L.C. (IP and Patent specialists)
http://kbpatentlaw.com/Pages/OurFirm.html




All previous medical device technologies such as "Topspin", "Smart Epidural" "Smart Ablation", etc. etc, we're under CooperVision, Inc. exclusivity under the pre-settlement Agreement. Now, EPGL has regained 100% control of those technologies too and plans to continue the patent prosecution for those technologies. This development adds even more potential IP back into EPGL hands as a result of the Settlement.
Company Summary
EP Global Communications, Inc., is a research and development company with focus on medical device design, ophthalmic medical device design, consumer electronic device design and device technologies in the IoT space. In 2014, all EPGL technologies and Intellectual property were selected and put under Development Agreement by CooperVision, Inc., a leading medical device manufacturer, for development and marketing. The EPGL team is now securing Intellectual Properties and co-developing a family of advanced devices as a result. This Development Agreement was publicly disclosed on August 29, 2014. In addition, EPGL has entered an initial Agreement with Intel Corporation. EPGL has also entered into Agreements with AT&T as of January 2015. EPGL and AT&T have created joint Intellectual Properties and are now in the process of procuring those properties.
EPGL Medical is the result of a reorganization which culminated in July 2012. With a strong team of medical and scientific professionals, the Company is poised to capture market share with innovative “smart medical and consumer electronic devices” technologies including those that require advanced microelectronic mechanical systems (MEMS). The Company philosophy is that healthcare is going to become an increasingly challenging environment for both physicians and patients. A coming boom in patients simultatneous to a possible shortage of physicians will necessitate intuitive medical devices which can be used by a broader variety of doctors. In short, new “smart medical devices” advanced research and design intellectual properties will be needed more than ever. EPGL Medical has the know-how to develop technologies which fit this coming new healthcare environment and enable industry leaders to capture market share with those advanced technologies.
Legal Counsel
Richard Medina Jr., Attorneys at Law
The Koll Center
501 W. Broadway, Suite 800
San Diego, CA, 92101
United States
Law Offices of Matthew Ladin
201 West Montecito Street
Santa Barbara, CA, 93101
United States
Michael Hayes & Dr. Markus at Intel HQ for meeting

Screenshot of Intel agreement
http://www.epglmed.com/EPGL_Medical_Sciences/Home_files/EPGLINTEL.png
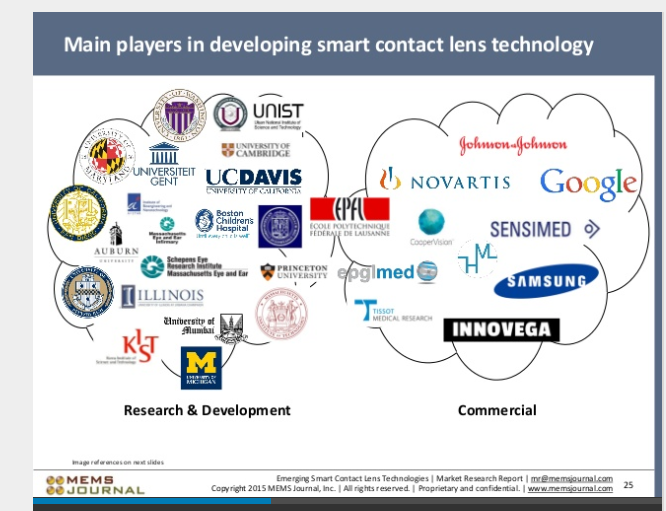
Independently Confirmed: MEMS Journal ranks $EPGL as a Major Player in upcoming smart contact revolution.
http://www.slideshare.net/MikePinelisPhD/smar...ort-sample

THE EPGL TEAM
Brady Peterson - IR/PR Manager
Brady Peterson was recently named Manager of Investor Relations and Public Relations as well as Information Technologies for EPGL Med. Mr. Peterson is the consummate professional who brings 15 years of Customer Relations and marketing experience to EPGL. The EPGL team is pleased to have him aboard and we believe customers and investors alike will appreciate his professionalism.
David T. Markus Ph.D. - Company Vice President - BioMems Development
David T. Markus holds a Ph.D. in Biomedical Engineering and a MS in Electrical Engineering with an emphasis in MEMS Microelectronics and Biomedical. Dr. Markus spent 11 years with Raytheon and holds 8 US patents and has 9 other US Patents Pending. He has been involved in research for several of the world's leading technological institutions, including the Office of Naval Research in Arlington, Virginia, NASA Jet Propulsion Laboratory in Pasadena, CA and he has been published 15 times for various technical conferences. He was a principal investigator on SBIR DARPA Phase I and Phase II, and "Ultraflexible Substrate" for Macroelectronics Program by Dr. Robert Reuss at DARPA. He has been involved in the engineering and the development on seven surgical devices, including devices for Cataract surgery, Intra-Ocular Lens Delivery, Arthroscopy, Endodontic Endoscope, Micro Endoscope and Neural Electrodes. Additionally he was instrumental on developing three medical laboratory devices, including for In-Vitro Fertilization, PCR instrumentation and drug discovery.
Eric Lee M.D.M.A.
Eric Lee M.D. M.A. graduated from Yale University and completed medical school at Boston University. He completed his residency in Physical Medicine and Rehabilitation at NYU Langone Medical Center where he has continued with a Pain Medicine fellowship. Dr. Lee is intimately familiar with the MPDD device and used it as a diagnostic tool in a study of using advanced techniques to treat myofascial pain along with Dr. Michel Dubois MD at NYU. Dr. Lee's professional interests include pain diagnosis and treatment, disorders of the spine, central, and peripheral nervous system, with a special interest in advanced interventional techniques and minimally invasive spinal procedures. Dr. Lee will oversee further additions to the EPGL Medical management team and advancement of new medical devices technologies the Company has interest in pursuing.
Ryan M. Stellar M.E. B.E.
EPGL is proud to have Ryan M. Stellar M.E. B.E. as part of the team. Mr. Stellar is a highly accomplished biomedical engineer who was intimately involved with the creation of the MPDD device as the Chief Engineer during its creation at Stevens Proof of Concept (SPOC). Mr. Stellar graduated from Stevens Institute of Technology in 2006 with a degree in Biomedical Engineering and a Minor in Economics. Mr. Stellar has been with Medtronic, Inc. for six years prior to leaving this year. While at Medtronic, among many other accomplishments, he successfully directed global launches of two portfolio critical products in the cardiac rhythm device market: DF4 Lead Connector System & CareLink Network for Heart Failure. Mr. Stellar is an expert in medical device manufacturing resources and distribution channels as well as customer relationship management.
Reid H. Erickson - Financial Partnership Strategy Advisor
Reid H. Erickson is Financial Partnership Strategy Advisor and candidate for Board of Directors. Reid is Founder, Chairman and CEO, Beneri International Corporation. He is an innovative, visionary leader spearheading green technology commercialization and the growth of sustainable businesses. With nearly three decades in the green and sustainable industry, Reid has assisted numerous early stage companies as an investor, advisor, consultant, board member or executive manager providing leadership and access to his worldwide network of entrepreneurial specialists dedicated to sustainable development.
Michael Hayes - Interim Company President & CEO
Michael Hayes was President of Digital Health Sciences, Inc. prior to that Company being acquired by EPGL in July 2012. Mr. Hayes has presided over the debt restructuring of EP Global Communications, Inc. along with Pricewaterhouse Coopers since early 2012. Mr. Hayes is now in charge of assembling a new management team for EPGL, including all Company management, medical and scientific personnel going forward. Mr. Hayes has made it a singular priority to bring aboard only the world’s top professionals in the field of medical sciences to EPGL and thereby build the Company into a major player in the medical device industry over the next several years. Mr. Hayes has also committed to prioritizing shareholders and creating significant value for their investments over time.
Noah T. Kaufman, M.D. - Vice President of Emergency Medicine Innovations
Noah T. Kaufman, M.D. as Vice President of Emergency Medicine Innovations and candidate for Board of Directors. Dr. Kaufman will add valuable energy and insight from the perspective of emergency medicine, which is a significant market for EPGL moving into the future. He is a highly accomplished physician in his field. As Director of Clinical Emergency Ultrasound, Dr. Kaufman is in the top ranks for patient scores and outcomes. Dr. Kaufman is also currently the attending Emergency Physician for Barton Memorial Hospital ER and the Carson Valley Medical Center ER at South Lake Tahoe, California. His education includes Akron General Medical Center, Akron, OH - EM Residency 2006. Tulane University School of Medicine, New Orleans, LA - MD 2003, and University of Miami, Coral Gables, FL - BS 1996
Andereas Markus - International Distributor
International Innovations is EPGL Medical Sciences exclusive new arm for marketing and distribution globally. Mr. Andereas Markus (M.B.A & M.Sc Eng) has both a strong Business and Engineering educational background. He is a talented and extremely well connected marketeer whose ability has helped him develop more than 20 years of experience in international marketing, engineering and distribution. This includes various high end products distributed to all of Asia, America, Europe, the Middle East and Africa. His professional experience has seen him working with all of the top 20 economic markets in the world.
As the new leader of International distribution for EPGL Medical Sciences, Andereas and his staff will treat physicians to a higher "VIP standard" of service, by listening carefully to what the doctor needs in medical device products, training and technical support. Andereas will implement EPGL's new and innovative approach to medical device marketing globally. This includes the willingness to make himself and EPGL staff available virtually 24/7 to service health care professionals and organizations in a timely manner. He will implement EPGL's new concierge style service to the world's top clients in health care from CEO's of international hospitals to front-line physicians and all between. This exceptional service and marketing model will ensure that health care professionals will have the best experience possible with EPGL Medical and its products. It is for these simple but powerful reasons that EPGL Medical Sciences will build a strong and loyal clientele worldwide for its new medical devices.
About EPGL Medical Science's Share Structure
EPGL has a share structure of 5 billion authorized shares with approximately 4.5 billion shares restricted and a float of approximately 499 million. Following a very successful Reverse Split EPGL has decreased the float by 90% and increased share holder value by over 500% since The company has strongly stated many times that they will not sell shares into the retail market. They are here to build a real company and implement real growth. Since restructuring EPGL has not increased the Float/OS/AS and have given us there word on countless occasions they will not do so.
Pricewaterhouse Coopers, Corporate Finance and Recovery (Cayman) Ltd.Controlling Agent for:
UPDATE**
AJW Qualified Partners, LLC,
2.0 Billion Common Shares->> Reduced to 1.975B
Mr. Michael Hayes
2.0 Billion Common Shares ->>Reduced to 1.975B
The proof Novartis/Alcon have been in contact with EPGL Medical.

MESSAGE FROM PricewaterhouseCopper Ian Stoake

Yes, Settlement has been Confirmed-> CooperVision/EPGL was validated by CooperVision IR.(BELOW)

Join the InvestorsHub Community
Register for free to join our community of investors and share your ideas. You will also get access to streaming quotes, interactive charts, trades, portfolio, live options flow and more tools.










