Wednesday, January 27, 2016 10:05:32 AM
Jan17-20 2016: NobleCon12 - Noble Financial's 12th Annual Emerging Growth Investor Conf., Port St. Lucie, FL
1-18-16/SK: “Delivering Innovative & Exceptional Biopharmaceutical Products to Improve Patient Lives”
REPLAY/21min. Video & 31 Slides (CEO Steve King): http://tinyurl.com/jr4vr36














SKing/8:50 Slide16 (1-18-16): “Unblinding at 80% of Events”






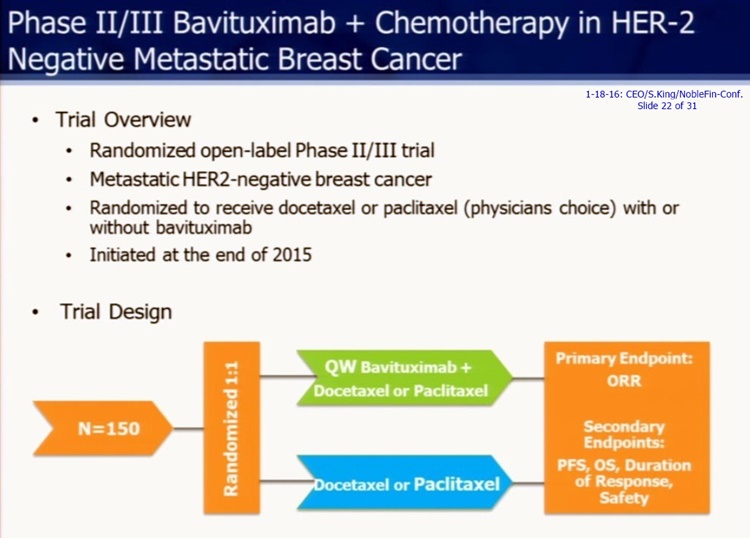









= = = = = = = = = = = = = = = = = = = = = = = = = = =
1-11-16/PR: Update on 4 New Bavi Clinical Trials (Lung/AZN, Breast/1Co./1MSKCC, Other Cancers/AZN). SUNRISE estimates: Interim1=Early'16, Interim2=Mid'16, FinalUnblinding=End'16… http://tinyurl.com/zhdy37a
1-6-16: Peregrine enters into Research Collab. with Natl-Comprehensive-Cancer-Network (NCCN) http://tinyurl.com/zmxtpsb
...$2mm res. grant to NCCN's Oncology Res. Pgm (ORP), will “significantly expand our clinical evaluation of Bavi and augment Peregrine's IST pgm at 26 of the world's leading cancer centers”.
12-10-15 Qtly. Conf. Call (King/Shan/Worsley/Garnick/Lytle) Transcript http://tinyurl.com/jkp885g
...CEO SK: “Although our SUNRISE enrollment milestone has been reached, we have no intention of slowing down, quite the opposite. We are aggressively moving to initiate new clinical trials [Lung, Breast, Mult-Types] that will allow us to build the most robust oncology business possible… With each of these studies our goal is the same - we are committed to identifying key indications, patient populations, and therapeutics that can benefit from combination treatment with bavituximab. From what we have seen to-date, the opportunity appears vast and we are hard at work converting the most promising prospects into true value.”
10-15-15 Peregrine's ASM: ATTENDEE Reports & Link to SK's 18min/16slide webcast: http://tinyurl.com/o6z4bm4
10-15-15: Peregrine & AstraZeneca Expand Collab. w/Ph2/2ndLine-NSCLC Trial, Bavi+durvalumab(MEDI4736), squamous or non-squamous. http://tinyurl.com/q79bkam
9-9-15 Qtly. Conf. Call (King/Shan/Worsley/Lytle) Transcript http://tinyurl.com/ph22vdn
...CEO S.King: “The Memorial Sloan Kettering & AstraZeneca collaborations are an important part of our announced plans to expand our bavituximab clinical pgm.”
8-24-15: AstraZeneca & Peregrine Collaborate on Bavi+Durvalumab Ph1/1B Trial for “multiple solid tumors” http://tinyurl.com/owlxpsf
...Durvalumab=MEDI4736(anti-PD-L1 immune checkpoint inhibitor). AZN’s Head/I-O(Robert Iannone): “Our partnership with Peregrine provides the opportunity to explore an exciting, novel combination that could deliver important clinical benefit to patients across a range of cancers."
7-14-15 Qtly. Conf. Call (King/Shan/Hutchins/Lytle) Transcript http://tinyurl.com/nw2v5u6
...CEO S.King: “We recently entered into collaboration with investigators at Memorial Sloan Kettering Cancer Ctr to continue expanding on this important work, as well as to explore other potential applications of bavituximab and other agents that target PS-signaling pathway.”
5-29-15: Peregrine & Sloan Kettering Enter Collab. to “Investigate Novel PS-Targeting Immunotherapy Combos” http://tinyurl.com/qxu4w2x
= = = = = = = = = = = = = = = = =RECALL:
12-10-15 Qtly. Conf. Call (King/Shan/Worsley/Garnick/Lytle) Transcript http://tinyurl.com/jkp885g
Q&A:
3. Rahul Jasuja - Noble Life Science Partners http://noblelsp.com/research
RJ: ”In planning your combination studies going forward, we are looking at PD-L1 low tumors because they are likely to be non-responsive to anti-PD-1's, like in Keytruda & Opdivo. Is that the only the rational, or is it also likely that PD-L1 low tumors also more responsive to bavituximab?”
Steve King: That’s what we want to show out in some of these studies we’re starting. We’re not planning on selecting for PD-L1 neg. patients in the Phase II study or the initial combination of bavi with chemo & durvalumab, but rather taking all-comers and then doing subset analysis and determining which patient populations we’re having the biggest impact in. Because, based on our translational data that’s been presented this year at ESMO & SITC'15 [11-9-15/SITC: http://tinyurl.com/pbof95w ], we’ve shown that we can take PD-L1 neg. tumors and actually elicit an immune response in those tumors. That’s the reason that we think that we have the potential to turn those into better responders on a PD-1 or PD-L1 therapy. As I mentioned during the prepared remarks it’s basically, “use bavi to get the immune response going and then use durvalumab to keep it going.” That's the reason there is a great scientific rationale right now for why we may have the biggest impact in those patients who don’t do well, because the delta between how they would normally do and how they might do with bavituximab may be the largest. We’re going to have some great insight into that from the studies we’re planning on running and the ability to go in and do subset analysis. Joe, do you want to add any more to that?
Joe Shan: I was going to use the word delta, but you beat me to it. I think in the PD-L1 neg. patient you have more opportunities to demonstrate the delta.
RJ: ”One of the concepts that’s evolving pretty rapidly is that you’ve got the TILs- & TILs+, and TILs+ are responding to PD-1 checkpoint immunotherapy. So is it fair to say, or is it an extrapolation, that PD-L1 positive tumors are more likely the ones that are TILs+? And in your case are you also seeing that TILs- tumors are probably the ones that are going to respond to chemo combination therapy better than the other ones that are the non-responders in combination with PS you can make them responders.”
Steve King: That’s certainly what our evidence has shown so far. The general assumption is that TILs- or TILs/Low tumors the ones that have low levels of the need for PD-L1, right, that’s really meant to stop an ongoing immune response. I think that’s generally true. Now it gets a little bit more complex because you’ve got Tregs and all kinds of T-cells present inside the tumor, so it also depends on the particular makeup. What we know is that when you get bavituximab, we seem to see a nice change in the levels of MDSCs (Myeloid-Derived Suppressor Cells) who are really the cell type that’s responsible for controlling the immune response in the tumor. It's been shown that patients with high levels of MDSCs have very poor prognosis. So as much as probably getting new TILs into the tumors is important, it’s probably more important to change the makeup of those cells into a more productive immune response positive phenotype. That’s exactly what we see with when you get bavituximab treatment; when we take a look a look at our translational data it shows an increase in CD4+ T-Cells and an increase in CD8+ T-Cells along with the changes in the suppressive cells types and the expression of immuno-suppressor cytokines, in which both decrease after treatment. So, it’s a matter of getting everything moving in the right direction. Again this is the role we think PS plays, by blocking it and activating an immune response, we're able to turn that around.
RJ: ”That does make sense. I think you’re looking at patient selection as being helpful in defining the population as well as the data you showedat SITC'15 [11-9-15/Duke's Herbert K. Lyerly http://tinyurl.com/pbof95w ], where you talked about immuno-profiling and looking at response to bavituximab, those are very interesting datasets. The other question I have is, there are a couple of trials running that are ISTs [at UTSW] - one of them is bavi in combination with Yervoy(ipilimumab) in melanoma, and the other one is a rectal cancer IST. Any updates on those?”
Steve King: The Rectal adenocarcinoma, we expect to have some data coming up this year from that study. As in any IST, it’s always a bit difficult, we do whatever we can to encourage them. But we do expect data to be becoming out of that study in 2016, which I think will be a real positive because in that study we did have the opportunity to collect pre- and post-treatment biopsies - #1, so we can see what happens following bavituximab treatment, but also that was a combination with radiation which we expect to be a very strong inducer of tumor antigens, which can then be taking advantage of by bavituximab treatment to make CD8+ T-Cells or killer T-Cells. For the Melanoma study, obviously the since that investigator (UTSW) started this study the SOC has changed pretty substantially. So right now, we're trying to work with that investigator, as well as some others, to probably change the profile of that study or start a new multi-center study in which we can then look at little bit more closely at what is the current SOC and make sure it’s an attractive trial for patients. As the treatment options for patient’s change, you need to be able to change with it and luckily it's a small IST trial and we may have an opportunity here to run a trial that I think would be very attractive for patients and will allow us to answer some key questions, because that was really the point of that study was to answer some of the key questions of combining with Yervoy. In addition, we’re starting the combination of durvalumab [AZN collab.] in multiple solid tumor types after the beginning of the year, so one way or another we’ll have lots of information coming from those studies.
RJ: ”Any updates any more color or comments on the collaboration with Sloan Kettering and Jed Wolchok on combination approaches - any data coming that way in the next few months?”
Steve King: That's an ongoing process. We’re very actively working with them to study a new combinations, looking for potential and different indications as well as those different combinations. We fully expect that it will be a fruitful collaboration; there will be a lots of data coming from that that we’ll see a very scientific meetings coming up. We already see a lot of data coming out of all for other collaborators nad we’ve had examples of presentations in multiple conferences this year. It always takes a while for them to get started, but then once they are going you tend to have a lot of data that continuously comes through them because the all the systems are up and running.
RJ:”Great. Thank you and congratulations on the healthy revenues at Avid.”
Recent CDMO News
- Avid Bioservices to Participate in Craig-Hallum Bioprocessing Conference • GlobeNewswire Inc. • 09/12/2024 08:05:27 PM
- Form 10-Q - Quarterly report [Sections 13 or 15(d)] • Edgar (US Regulatory) • 09/09/2024 08:43:56 PM
- Form 8-K - Current report • Edgar (US Regulatory) • 09/09/2024 08:19:30 PM
- Avid Bioservices Reports Financial Results for First Quarter Ended July 31, 2024 • GlobeNewswire Inc. • 09/09/2024 08:05:31 PM
- U.S. Futures Rise Amid Inflation Report Anticipation; Oil Prices Climb on Hurricane Threat and Supply Concerns • IH Market News • 09/09/2024 10:09:14 AM
- Avid Bioservices to Report Financial Results for First Quarter of Fiscal Year 2025 After Market Close on September 9, 2024 • GlobeNewswire Inc. • 09/03/2024 08:05:20 PM
- Form 8-K - Current report • Edgar (US Regulatory) • 08/29/2024 08:30:10 PM
- Form ARS - Annual Report to Security Holders • Edgar (US Regulatory) • 08/28/2024 08:34:04 PM
- Form DEFA14A - Additional definitive proxy soliciting materials and Rule 14(a)(12) material • Edgar (US Regulatory) • 08/28/2024 08:32:18 PM
- Form DEF 14A - Other definitive proxy statements • Edgar (US Regulatory) • 08/28/2024 08:30:28 PM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 07/16/2024 11:50:20 PM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 07/16/2024 11:48:19 PM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 07/15/2024 08:40:04 PM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 07/12/2024 08:30:04 PM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 07/11/2024 09:02:39 PM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 07/11/2024 09:02:27 PM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 07/11/2024 09:01:22 PM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 07/11/2024 09:01:05 PM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 07/11/2024 09:00:54 PM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 07/11/2024 09:00:45 PM
- Form 144 - Report of proposed sale of securities • Edgar (US Regulatory) • 07/11/2024 12:23:26 AM
- Avid Bioservices Reports Financial Results for Fourth Quarter and Fiscal Year Ended April 30, 2024 • GlobeNewswire Inc. • 07/02/2024 08:05:04 PM
- Avid Bioservices to Report Financial Results for Quarter and Fiscal Year Ended April 30, 2024, After Market Close on July 2, 2024 • GlobeNewswire Inc. • 07/01/2024 11:00:21 AM
- Avid Bioservices Earns Committed Badge from EcoVadis for Sustainability Performance • GlobeNewswire Inc. • 05/23/2024 12:05:46 PM
- Avid Bioservices to Participate at Upcoming Investor Conferences • GlobeNewswire Inc. • 05/07/2024 08:05:11 PM
VHAI - Vocodia Partners with Leading Political Super PACs to Revolutionize Fundraising Efforts • VHAI • Sep 19, 2024 11:48 AM
Dear Cashmere Group Holding Co. AKA Swifty Global Signs Binding Letter of Intent to be Acquired by Signing Day Sports • DRCR • Sep 19, 2024 10:26 AM
HealthLynked Launches Virtual Urgent Care Through Partnership with Lyric Health. • HLYK • Sep 19, 2024 8:00 AM
Element79 Gold Corp. Appoints Kevin Arias as Advisor to the Board of Directors, Strengthening Strategic Leadership • ELMGF • Sep 18, 2024 10:29 AM
Mawson Finland Limited Further Expands the Known Mineralized Zones at Rajapalot: Palokas step-out drills 7 metres @ 9.1 g/t gold & 706 ppm cobalt • MFL • Sep 17, 2024 9:02 AM
PickleJar Announces Integration With OptCulture to Deliver Holistic Fan Experiences at Venue Point of Sale • PKLE • Sep 17, 2024 8:00 AM







