Sunday, November 29, 2015 10:43:32 AM
$GHTP Highlights:
Share Structure:
500M AS 144M OS
Upcoming Catalysts:
Meeting with FDA on Monday November 30th
Revenues:
$GTHP guaranteed $14mm in sales for their LuViva patented technology over next 4 years
Guided Therapeutics’ Fourth Quarter Orders of LuViva® Advanced Cervical Scan Disposables Triple 2014 Total Sales
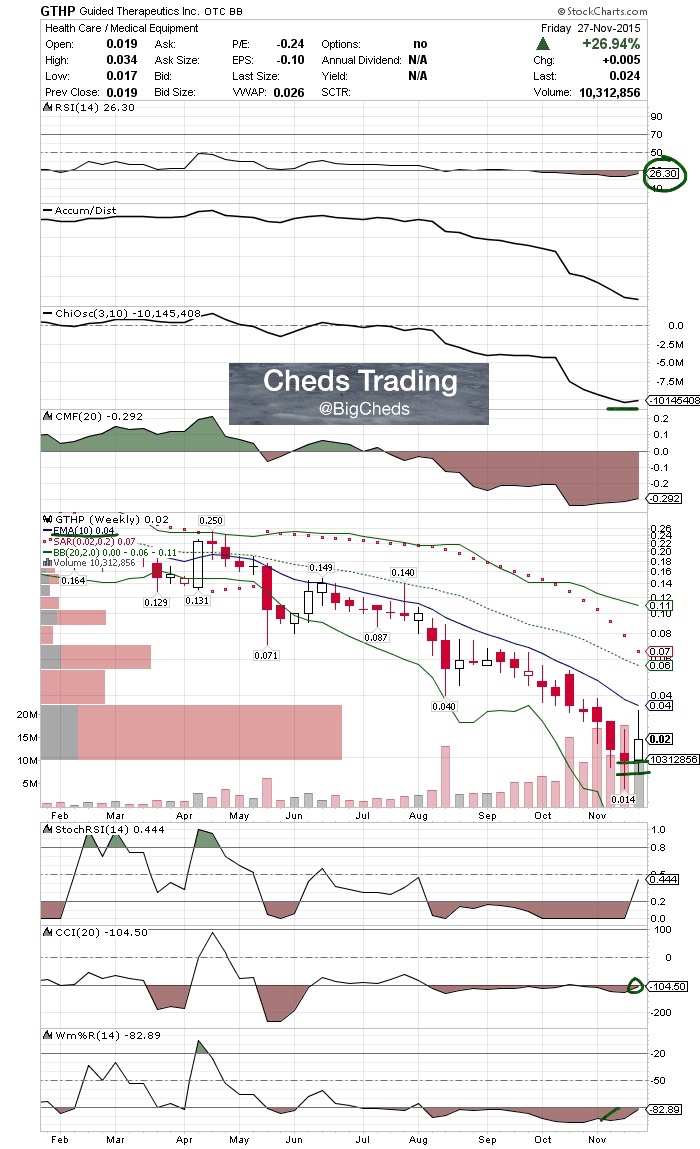
Chart:
$GTHP - CCI nearing cross, williams oversold and RSI sub 30s. Severely oversold and great spot for entry.
Extended DD:
http://www.otcmarkets.com/stock/GTHP/news
http://www.otcmarkets.com/stock/GTHP/financials
$GTHP guaranteed $14mm in sales for their LuViva patented technology over next 4 years:
In july 2015 Turkish Distributor signed a four year deal to sell $10 million of LuViva Devices to the Turkish Ministry of Heath, and has expanded this order to $14 million.

Meeting with FDA on Monday November 30th
http://seekingalpha.com/news/2902666-guided-therapeutics-to-meet-with-fda-on-november-30-to-clarify-requirements-for-pma-amendment-for-luviva-system
The FDA agrees to meet with struggling nano cap Guided Therapeutics (OTCQB:GTHP +0.7%) on November 30 instead of November 6 to determine the specific additional patient data the agency requires for the company's Premarket Approval (PMA) application for its LuViva Advanced Cervical Scan system. The date change was to accommodate the schedules of two key clinical investigators
The company has been unable to gain clearance in the U.S. for LuViva. It received its first Complete Response Letter (CRL) about two years ago and a second CRL in May. A CRL is the official response from the agency stating the PMA is not approvable in its present form.
LuViva, designed to rapidly detect cervical pre-cancer without the need for a tissue sample, is currently cleared for sale in Europe, Canada and Mexico.
FDA Meeting Press Release
FDA Grants Guided Therapeutics’ Request for New Meeting Date to Plan Path Forward for LuViva® Advanced Cervical Scan PMA Application
Nov 05, 2015
OTC Disclosure & News Service
-
Guided Therapeutics, Inc. (OTCQB: GTHP), the maker of a rapid and painless testing platform based on its patented biophotonic technology, announced today the U.S. Food and Drug Administration (FDA) has set a new meeting date of November 30, 2015, to review the company’s plan to submit an approvable application for the LuViva® Advanced Cervical Scan.
The company requested the new date to accommodate the schedules of two physicians who plan to attend the meeting on the company’s behalf. Dr. Leo B. Twiggs, professor emeritus at the Miller School of Medicine, University of Miami and Dr. Daron Ferris, professor at the Medical College of Georgia are principal investigators of the LuViva pivotal clinical trial. Both are past presidents of the American Society of Colposcopy and Cervical Pathology.
“We appreciate the agency working with us to accommodate the busy schedules of Drs. Ferris and Twiggs,” said Gene Cartwright, CEO of Guided Therapeutics. “Having them both at the meeting will make it more productive and hopefully lead to the most positive outcome.”
The LuViva is designed to detect cervical pre-cancer without taking a tissue sample and providing a result immediately, unlike any other women’s health cancer detection technology on the market. The LuViva is approved in Europe, Canada and Mexico and is currently available to women in 20 countries.
The FDA, in a not approvable letter, recommended that the company include additional patient data as part of its next PMA amendment. A key objective of the scheduled meeting will be to determine the parameters of the additional patient data FDA is seeking.
World-wide, the market for cervical cancer screening and diagnostics, as currently practiced using cytology (Pap test) for primary screening, is estimated at $6 billion and is projected to grow to almost $9 billion by 2020. Worldwide there are about 2.6 billion women aged 15 years and older who are at risk of developing cervical cancer.
Guided Therapeutics’ Fourth Quarter Orders of LuViva® Advanced Cervical Scan Disposables Triple 2014 Total Sales
http://www.otcmarkets.com/stock/GTHP/news/Guided-Therapeutics-rsquo--Fourth-Quarter-Orders-of-LuViva-reg--Advanced-Cervical-Scan-Disposables-Triple-2014-Total-Sales?id=116857&b=y
Guided Therapeutics, Inc. (OTCQB: GTHP), the maker of a rapid and painless testing platform based on its patented biophotonic technology, announced today that it expects to ship 25 LuViva® Advance Cervical Scan units and approximately 40,000 single-patient-use disposables in the fourth quarter of 2015.
The shipments are destined for Turkey, Saudi Arabia and Indonesia with manufacturing underway at the Guided Therapeutics facility in Norcross, Georgia.
For the year, the company expects to have shipped 45 LuViva units and approximately 45,000 disposables generating revenue of $1.1 million. This compares to 41 units and approximately 15,000 disposables in 2014 for revenue of $758,000.
“The tremendous burst of sales in the fourth quarter is reflective of closing the Turkish Ministry of Health order and shows the opportunity for growth based on our strategy of working with governments,” said Gene Cartwright, CEO of Guided Therapeutics. “Our primary targets are countries that need a point-of-care solution for cervical cancer screening of large underserved populations.”
Worldwide, the market for cervical cancer screening and diagnostics, as currently practiced using cytology (Pap test) for primary screening, is estimated at $6 billion and is projected to grow to almost $9 billion by 2020. There are about 2.6 billion women aged 15 years and older who are at risk of developing cervical cancer worldwide.
Chart:
$GTHP - CCI nearing cross, williams oversold and RSI sub 30s. Meeting FDA Monday - 500M AS - 14m guaranteed in revs
Share structure:
500M AS 144M OS
Share Structure:
500M AS 144M OS
Upcoming Catalysts:
Meeting with FDA on Monday November 30th
Revenues:
$GTHP guaranteed $14mm in sales for their LuViva patented technology over next 4 years
Guided Therapeutics’ Fourth Quarter Orders of LuViva® Advanced Cervical Scan Disposables Triple 2014 Total Sales
Chart:
$GTHP - CCI nearing cross, williams oversold and RSI sub 30s. Severely oversold and great spot for entry.
Extended DD:
http://www.otcmarkets.com/stock/GTHP/news
http://www.otcmarkets.com/stock/GTHP/financials
$GTHP guaranteed $14mm in sales for their LuViva patented technology over next 4 years:
In july 2015 Turkish Distributor signed a four year deal to sell $10 million of LuViva Devices to the Turkish Ministry of Heath, and has expanded this order to $14 million.

Meeting with FDA on Monday November 30th
http://seekingalpha.com/news/2902666-guided-therapeutics-to-meet-with-fda-on-november-30-to-clarify-requirements-for-pma-amendment-for-luviva-system
The FDA agrees to meet with struggling nano cap Guided Therapeutics (OTCQB:GTHP +0.7%) on November 30 instead of November 6 to determine the specific additional patient data the agency requires for the company's Premarket Approval (PMA) application for its LuViva Advanced Cervical Scan system. The date change was to accommodate the schedules of two key clinical investigators
The company has been unable to gain clearance in the U.S. for LuViva. It received its first Complete Response Letter (CRL) about two years ago and a second CRL in May. A CRL is the official response from the agency stating the PMA is not approvable in its present form.
LuViva, designed to rapidly detect cervical pre-cancer without the need for a tissue sample, is currently cleared for sale in Europe, Canada and Mexico.
FDA Meeting Press Release
FDA Grants Guided Therapeutics’ Request for New Meeting Date to Plan Path Forward for LuViva® Advanced Cervical Scan PMA Application
Nov 05, 2015
OTC Disclosure & News Service
-
Guided Therapeutics, Inc. (OTCQB: GTHP), the maker of a rapid and painless testing platform based on its patented biophotonic technology, announced today the U.S. Food and Drug Administration (FDA) has set a new meeting date of November 30, 2015, to review the company’s plan to submit an approvable application for the LuViva® Advanced Cervical Scan.
The company requested the new date to accommodate the schedules of two physicians who plan to attend the meeting on the company’s behalf. Dr. Leo B. Twiggs, professor emeritus at the Miller School of Medicine, University of Miami and Dr. Daron Ferris, professor at the Medical College of Georgia are principal investigators of the LuViva pivotal clinical trial. Both are past presidents of the American Society of Colposcopy and Cervical Pathology.
“We appreciate the agency working with us to accommodate the busy schedules of Drs. Ferris and Twiggs,” said Gene Cartwright, CEO of Guided Therapeutics. “Having them both at the meeting will make it more productive and hopefully lead to the most positive outcome.”
The LuViva is designed to detect cervical pre-cancer without taking a tissue sample and providing a result immediately, unlike any other women’s health cancer detection technology on the market. The LuViva is approved in Europe, Canada and Mexico and is currently available to women in 20 countries.
The FDA, in a not approvable letter, recommended that the company include additional patient data as part of its next PMA amendment. A key objective of the scheduled meeting will be to determine the parameters of the additional patient data FDA is seeking.
World-wide, the market for cervical cancer screening and diagnostics, as currently practiced using cytology (Pap test) for primary screening, is estimated at $6 billion and is projected to grow to almost $9 billion by 2020. Worldwide there are about 2.6 billion women aged 15 years and older who are at risk of developing cervical cancer.
Guided Therapeutics’ Fourth Quarter Orders of LuViva® Advanced Cervical Scan Disposables Triple 2014 Total Sales
http://www.otcmarkets.com/stock/GTHP/news/Guided-Therapeutics-rsquo--Fourth-Quarter-Orders-of-LuViva-reg--Advanced-Cervical-Scan-Disposables-Triple-2014-Total-Sales?id=116857&b=y
Guided Therapeutics, Inc. (OTCQB: GTHP), the maker of a rapid and painless testing platform based on its patented biophotonic technology, announced today that it expects to ship 25 LuViva® Advance Cervical Scan units and approximately 40,000 single-patient-use disposables in the fourth quarter of 2015.
The shipments are destined for Turkey, Saudi Arabia and Indonesia with manufacturing underway at the Guided Therapeutics facility in Norcross, Georgia.
For the year, the company expects to have shipped 45 LuViva units and approximately 45,000 disposables generating revenue of $1.1 million. This compares to 41 units and approximately 15,000 disposables in 2014 for revenue of $758,000.
“The tremendous burst of sales in the fourth quarter is reflective of closing the Turkish Ministry of Health order and shows the opportunity for growth based on our strategy of working with governments,” said Gene Cartwright, CEO of Guided Therapeutics. “Our primary targets are countries that need a point-of-care solution for cervical cancer screening of large underserved populations.”
Worldwide, the market for cervical cancer screening and diagnostics, as currently practiced using cytology (Pap test) for primary screening, is estimated at $6 billion and is projected to grow to almost $9 billion by 2020. There are about 2.6 billion women aged 15 years and older who are at risk of developing cervical cancer worldwide.
Chart:
$GTHP - CCI nearing cross, williams oversold and RSI sub 30s. Meeting FDA Monday - 500M AS - 14m guaranteed in revs

Share structure:
500M AS 144M OS

* May your trades turn out well and bring you profit - Good luck *
Join the InvestorsHub Community
Register for free to join our community of investors and share your ideas. You will also get access to streaming quotes, interactive charts, trades, portfolio, live options flow and more tools.






