Friday, January 30, 2015 12:00:00 PM
1-30-15 MLV Peregrine Company Update: ”Phase II Liver Cancer Trial of Bavituximab + Sorafenib Meets Primary Endpoint of mTTP” (George B. Zavoico)
https://mlv.bluematrix.com/sellside/EmailDocViewer?encrypt=3a98541b-c0bb-457b-a3ed-81855ce5ceb7&mime=pdf
PAGE 1 of 11 (see remainder below):
We note that a non-randomized, single-arm Phase II trial of bavituximab in combination with sorafenib (Nexavar®) for the treatment of advanced hepatocellular carcinoma (HCC) met its primary endpoint of prolonging the median time to radiologic progression (mTTP) compared to historical data. The combination was also safe and well-tolerated. However, the improved mTTP did not translate into an improvement in median overall survival (mOS), a secondary endpoint, most likely due to the small size of the trial (n=38) and early deaths unrelated to disease progression. We believe that the prolongation of mTTP is a signal of efficacy that supports our view that bavituximab is an active drug. We conclude that further evaluation is warranted in a larger, randomized trial. We maintain our BUY rating and $3.50 price target for Peregrine Pharmaceuticals, Inc.
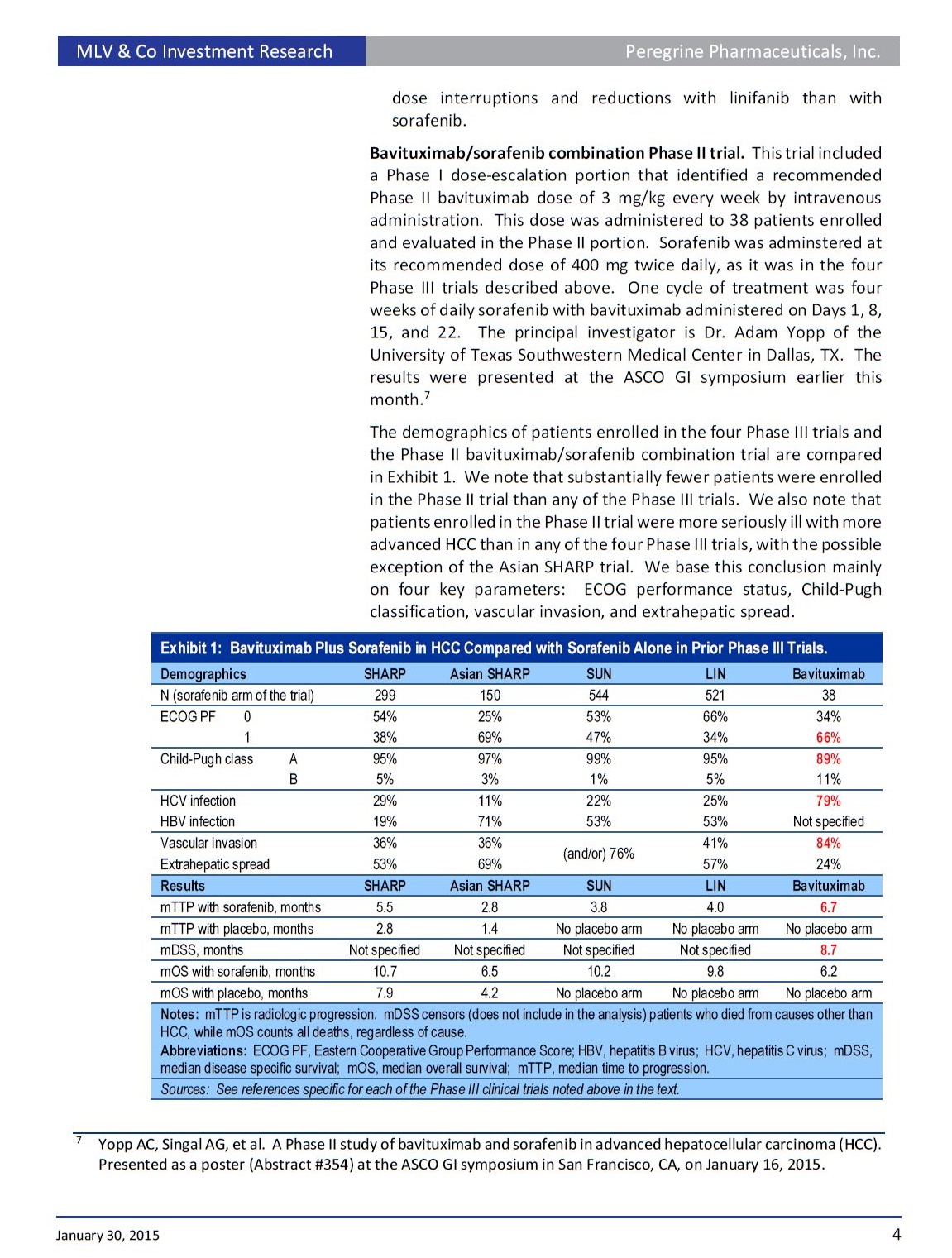
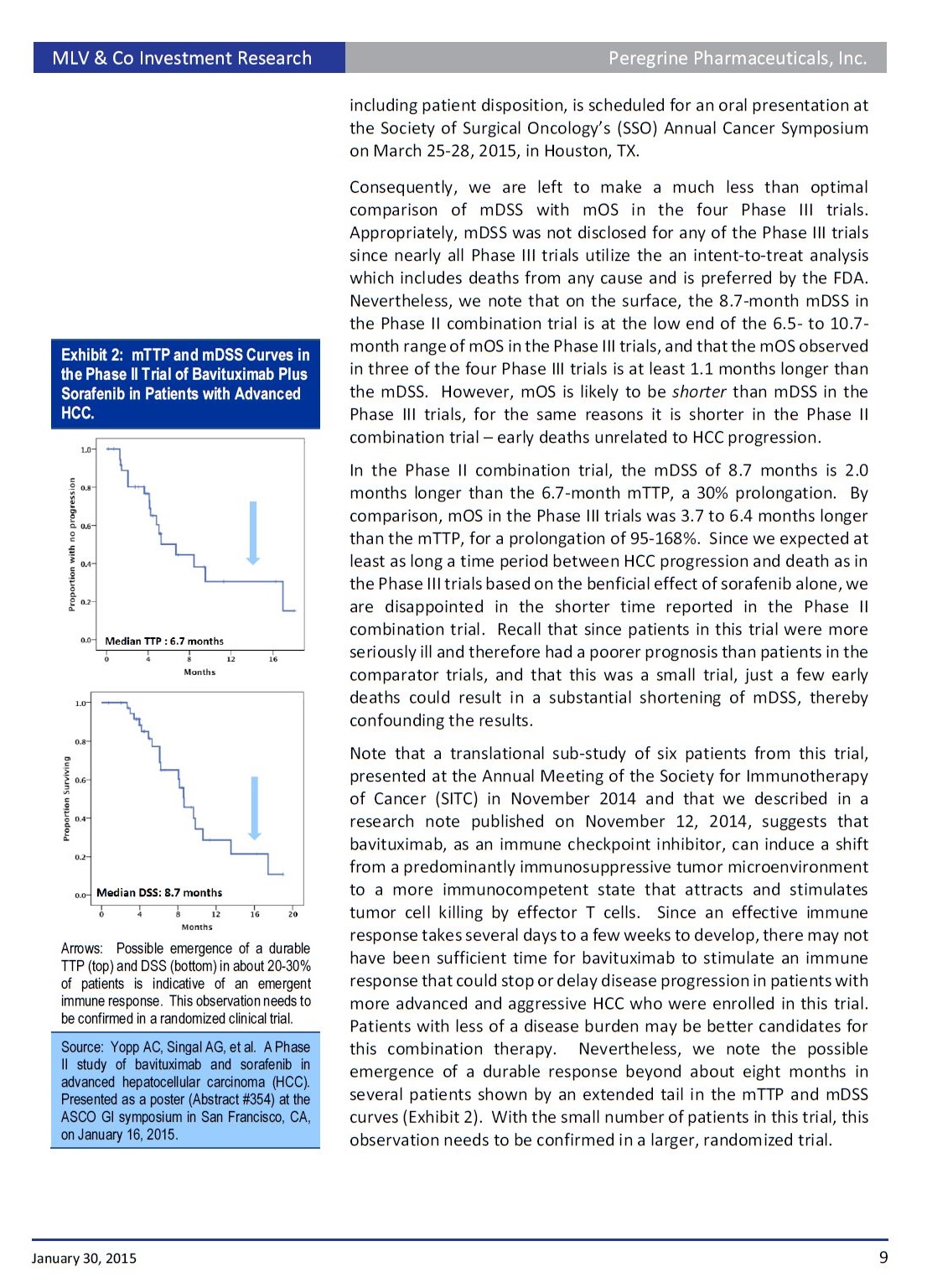
• Results. As reported at the ASCO-Gastrointestinal Cancers symposium on January 16, a mTTP of 6.7 months was reported for the bavituximab/sorafenib combination. This is a 1.2 to 3.9-month improvement over a range of 2.8 to 5.5 months for sorafenib alone reported in four prior Phase III trials. The median disease specific survival (mDSS), a secondary endpoint, was 8.7 months. These promising results were confounded by a median overall survival (mOS), another secondary endpoint, of 6.2 months, which is outside the 6.5 to 10.7- month range reported in prior trials for sorafenib alone.
• Protocol and Demographics. This investigator-sponsored, open label Phase I/II trial (ClinicalTrials.gov<http://clinicaltrials.gov/>; identifier: NCT01264705) enrolled patients with advanced HCC not eligible for resection or transplantation who were at least four weeks removed from prior locoregional therapies. Patients with metastatic disease were allowed. Notably, patients in this trial were more seriously ill than in prior trials of sorafenib alone based on the proportion of patients with Child-Pugh A score, ECOG performance status 0, and macrovascular invasion (MVI). However, fewer patients in this trial presented with extrahepatic spread (metastases) than in prior trials.
• Medical History. HCC is a heterogeneous disease, complicated and exacerbated by comorbidities such as hepatitis B or C (HBV or HCV) infection, alcohol abuse, underlying cirrhosis, and/or nonalcoholic steatohepatitis (NASH). Response to therapy can vary, depending on severity of disease, comorbidities, and medical history. Patients in different geographic regions are also known to have different prognoses. This heterogeneity underscores the necessity of conducting large enough randomized trials with well defined eligibility criteria to properly compare different therapeutic regimens.
• Summary. Since the adverse event profile of the bavituximab/sorafenib combination was not markedly different than with sorafenib alone, we conclude that this combination is as safe and well tolerated as sorafenib. While we would have liked to see a longer mOS with the combination, we are nevertheless encouraged by the improved mTTP for this difficult to treat patient population for which sorafenib is the only approved therapy. We think the results of this trial justify a larger and more rigorously designed and randomized Phase IIb trial to determine if the combination is superior to sorafenib alone in this indication.
MLV/George B. Zavoico - init. 2-8-10 Buy/PT=$10, CURR: Buy/$3.50 http://www.mlvco.com








Pgs 12-14: Disclosures & MLV Corp. Info.
= = = = = = = = = = = = = = = = = = = = = = = = = = =
H. 1st Investigator-Sponsored (IST) Ph.I/II Trial (Bavi+Sorafenib vs. Liver Cancer/HCC, open-label, n=9+38=47)
...Note: Sorafenib = Onyx/Bayer's Nexavar - see http://www.nexavar.com
Protocol: http://clinicaltrials.gov/ct2/show/NCT01264705 UTSW: http://tinyurl.com/mwdc2ql (5 sites: 3/UTSW, Parkland-Hosp, Dallas/VA, PI=Dr. Adam Yopp)
...1-16-15 ASCO Gastro-Symposium: Ph.2 data/n=38 (Adam Yopp), “These clinical outcomes of TTP=6.7/DCR=58%/PFS=4mo are quite encouraging…” http://tinyurl.com/m9uz9mo
…11-8-14: SITC'14 Ph.2 Correlative Studies data (biopsies B4/After) on 6pts, incl. KOL Dimitry Gabrilovich’s comments: http://tinyurl.com/pchzr6h
...9-9-14 Enrollment complete. http://tinyurl.com/ktrfswj (ph2=38 Ph1=9)
...4-4-12 AACR'12: Dr. Adam Yopp, "promising safety profile to-date" http://tinyurl.com/7yrwqm7 (see #5591)
...Feb'12-Sep'14 10+ times: CEO Steve King hints of future ex-US partner-driven Bavi+Sorafenib/LIVER trial in Asia-Pacific: http://tinyurl.com/nkaxtcc
......Articles & Data describe Liver Cancer challenges in Asian populations: http://tinyurl.com/7z7o8j9 & http://tinyurl.com/7z99cy4
...12-1-10: PPHM's 1st IST (Liver Cancer) initiated at UTSW, ~56 patients - http://tinyurl.com/3xd3e6c
…Per S.King, 5-18-10/R&R, "We've had a lot of interest in running clinical trials with the compound from investigators who have either had prior experience with the drug or would like to study the drug in various settings. Potential IST indications include all the major solid tumor types. Of particular interest is Liver Cancer, in which we have a natural tie-in with our HCV program, Ovarian Cancer and Pancreatic Cancer, also very nicely supported by the prior data."
= = = = = = = = = = = = = = = = = =
1-16-14/PR: Bavi+Sorafenib LIVER IST Ph2-data at ASCO Gastrointestinal Cancers Symposium in SanFran. Dr. Adam Yopp (UTSW), "These clinical outcomes of TTP, DCR, and 4-mo. PFS are quite encouraging, especially in this heavily pretreated patient cohort with very poor prognosis due to their unfavorable disease biology including a high rate of macrovascular invasion. I was also pleased to see an extended tail in the survival curve that is typical of emerging immunotherapies for cancer…”.
1-16-15: Phase II Clinical Data of Peregrine Pharmaceuticals' Bavituximab in Combination With Sorafenib Presented at ASCO Gastrointestinal Cancers Symposium
• Positive Trends in Time to Progression, Disease Control Rates and 4-Month Progression-Free Survival Endpoints Were Reported in a Patient Population With Poor Prognosis
• Survival Curves Showed Patients With Prolonged Survival Consistent With Those Seen in Other Immunotherapy Studies in Different Indications
• Overall Data From Trial Support Bavituximab's Immunostimulatory Mechanism-of-Action
• http://ir.peregrineinc.com/releasedetail.cfm?ReleaseID=891707
TUSTIN 1/16/15: Peregrine Pharmaceuticals, Inc. (NASDAQ: PPHM) (NASDAQ: PPHMP), today announced the presentation of clinical data related to the company's immuno-oncology development program and its lead investigational immunotherapy drug candidate bavituximab at the American Society of Clinical Oncology (ASCO) Gastrointestinal Cancers Symposium. This conference is being held January 15-17, 2015 at the Moscone West Convention Center in San Francisco, California.
The clinical presentation titled: "A Phase II Study of Bavituximab and Sorafenib in Advanced Hepatocellular Carcinoma (HCC)" will be presented this afternoon by Adam Yopp, M.D., assistant professor of surgery at the University of Texas Southwestern Medical Center Dallas, Texas.
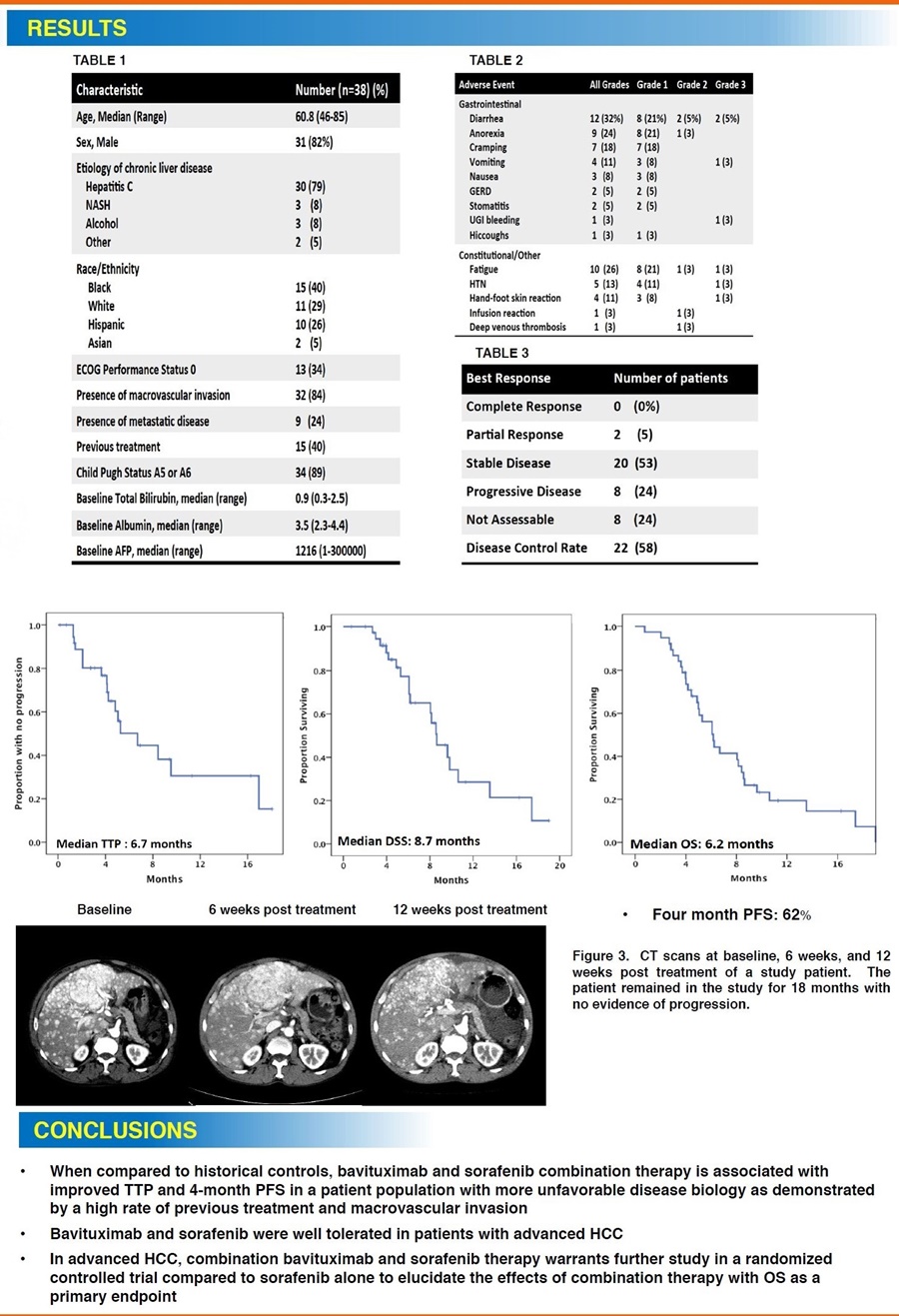
In this single-center, single-arm, open-label investigator-sponsored trial (IST), 38 patients with advanced HCC received bavituximab (3mg/kg) weekly and sorafenib (400 mg) twice daily until disease progression or toxicity. Data show that the combination of bavituximab and sorafenib is associated with an improved time to progression (TTP) of 6.7 months, a disease specific survival (DSS) of 8.7 months, a disease control rate (DCR) of 58% (22 out of 38 patients) and a 4-month progression-free survival (PFS) of 62%. Two patients (5%) achieved a partial response according to Response Evaluation Criteria In Solid Tumors (RECIST). The secondary endpoint of median overall survival (OS) was 6.2 months. The combination of bavituximab and sorafenib was well-tolerated in patients with advanced HCC with no indications of autoimmune adverse events that have been seen with other checkpoint immunotherapies.
"These clinical outcomes of time to progression, disease control rate and 4-month progression-free survival are quite encouraging, especially in this heavily pretreated patient cohort with very poor prognosis due to their unfavorable disease biology including a high rate of macrovascular invasion," said Dr. Yopp. "I was also pleased to see an extended tail in the survival curve that is typical of emerging immunotherapies for cancer. The positive data from this study should be considered as rationale for future randomized trials to further evaluate the potential of bavituximab in liver cancer."
"These data, along with recently reported translational data from this study, continue to build the knowledge base for the bavituximab clinical program and, in particular, highlight the potential immunotherapeutic synergies of bavituximab and sorafenib. We agree with Dr. Yopp that these data warrant further clinical evaluation of this combination in later stage clinical trials," said Joseph Shan, VP of Clinical & Regulatory for Peregrine. "We continue to build value in the bavituximab program across multiple programs including the execution of the SUNRISE Phase III trial in second-line, non-small cell lung cancer and from data generated from this and other investigator-sponsored trials as well as other ongoing clinical trials. We look forward to new clinical collaborations with the goal of further exploring the utility of bavituximab in combination with other immuno-oncology drugs."
A link to today's poster can be found from the front page of the company's website at: http://www.peregrineinc.com .
[ http://www.peregrineinc.com/images/stories/pdfs/gi_yopp_2015.pdf ]
Liver IST Clinical Translation Data
Recently presented translational data of 6 patients from this trial show that half of the patients evaluated had an increase in tumor fighting immune cells following one cycle of bavituximab treatment, similar to what has been shown for PS-targeting antibodies in multiple preclinical cancer models. In addition, the increase in immune response was associated with patients that remained on study treatment for longer time periods, suggesting the possibility of a clinically meaningful anti-tumor immune response. Three of the six patients evaluated had increased infiltration of activated tumor-fighting T-cells (CD8) into the tumor microenvironment which correlated with a prolonged time to disease progression. In addition, these responding patients initially expressed lower levels of PD-1 positive cells, an established marker of T-cell activation and disease outcome, prior to the initiation of therapy that was followed by a measurable rise post bavituximab treatment.
About the Phase II Trial
In this Phase II non-randomized, open-label trial, 38 patients with advanced HCC received bavituximab weekly and sorafenib (400 mg) twice daily, until disease progression or toxicity. The primary endpoint of this trial is radiologic time to progression with imaging occurring at 6 week intervals. Secondary endpoints of this trial include overall survival (OS), progression free survival (PFS), safety and response rates. In order to leverage recent understandings surrounding the immune-stimulatory mechanism of action of bavituximab, several additional components have been installed into this portion of the trial. These include plasma and serum collection and tissue biopsies for evaluating changes in immune response following bavituximab treatment to assess whether combination therapy reactivates tumor immunity by changing the tumor microenvironment from immunosuppressive to immunoreactive by changing the tumor infiltrating cell composition or inducing T-cell response to tumor antigens.
More information on this trial can be found at ClinicalTrials.gov using the Identifier NCT01264705. [ https://clinicaltrials.gov/ct2/show/NCT01264705 ]
ABOUT PEREGRINE PHARMACEUTICALS, INC.
Peregrine Pharmaceuticals, Inc. is a biopharmaceutical company with a pipeline of novel drug candidates in clinical trials for the treatment and diagnosis of cancer. The company's lead immunotherapy candidate, bavituximab is in Phase III development for the treatment of second-line non-small lung cancer (the "SUNRISE trial") along with several investigator-sponsored trials evaluating other treatment combinations and additional oncology indications. The company is also advancing a molecular imaging agent, 124I-PGN650, in an exploratory clinical trial for the imaging of multiple solid tumor types. Peregrine also has in-house cGMP manufacturing capabilities through its wholly-owned subsidiary Avid Bioservices, Inc. ( http://www.avidbio.com ), which provides development and biomanufacturing services for both Peregrine and third-party customers. Additional information about Peregrine can be found at http://www.peregrineinc.com .
Safe Harbor *snip*
Contact: Christopher Keenan, Peregrine Pharmaceuticals, Inc., 800-987-8256, info@peregrineinc.com
- - - - - - - - - - - - - - - -
Dr. Adam Yopp’s (UTSW) 1-16-2015 LIVER Poster (ASCO Gastrointestinal Cancers Symposium):
http://www.peregrineinc.com .
http://www.peregrineinc.com/images/stories/pdfs/gi_yopp_2015.pdf

Recent CDMO News
- Avid Bioservices to Participate in Craig-Hallum Bioprocessing Conference • GlobeNewswire Inc. • 09/12/2024 08:05:27 PM
- Form 10-Q - Quarterly report [Sections 13 or 15(d)] • Edgar (US Regulatory) • 09/09/2024 08:43:56 PM
- Form 8-K - Current report • Edgar (US Regulatory) • 09/09/2024 08:19:30 PM
- Avid Bioservices Reports Financial Results for First Quarter Ended July 31, 2024 • GlobeNewswire Inc. • 09/09/2024 08:05:31 PM
- U.S. Futures Rise Amid Inflation Report Anticipation; Oil Prices Climb on Hurricane Threat and Supply Concerns • IH Market News • 09/09/2024 10:09:14 AM
- Avid Bioservices to Report Financial Results for First Quarter of Fiscal Year 2025 After Market Close on September 9, 2024 • GlobeNewswire Inc. • 09/03/2024 08:05:20 PM
- Form 8-K - Current report • Edgar (US Regulatory) • 08/29/2024 08:30:10 PM
- Form ARS - Annual Report to Security Holders • Edgar (US Regulatory) • 08/28/2024 08:34:04 PM
- Form DEFA14A - Additional definitive proxy soliciting materials and Rule 14(a)(12) material • Edgar (US Regulatory) • 08/28/2024 08:32:18 PM
- Form DEF 14A - Other definitive proxy statements • Edgar (US Regulatory) • 08/28/2024 08:30:28 PM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 07/16/2024 11:50:20 PM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 07/16/2024 11:48:19 PM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 07/15/2024 08:40:04 PM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 07/12/2024 08:30:04 PM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 07/11/2024 09:02:39 PM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 07/11/2024 09:02:27 PM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 07/11/2024 09:01:22 PM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 07/11/2024 09:01:05 PM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 07/11/2024 09:00:54 PM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 07/11/2024 09:00:45 PM
- Form 144 - Report of proposed sale of securities • Edgar (US Regulatory) • 07/11/2024 12:23:26 AM
- Avid Bioservices Reports Financial Results for Fourth Quarter and Fiscal Year Ended April 30, 2024 • GlobeNewswire Inc. • 07/02/2024 08:05:04 PM
- Avid Bioservices to Report Financial Results for Quarter and Fiscal Year Ended April 30, 2024, After Market Close on July 2, 2024 • GlobeNewswire Inc. • 07/01/2024 11:00:21 AM
- Avid Bioservices Earns Committed Badge from EcoVadis for Sustainability Performance • GlobeNewswire Inc. • 05/23/2024 12:05:46 PM
- Avid Bioservices to Participate at Upcoming Investor Conferences • GlobeNewswire Inc. • 05/07/2024 08:05:11 PM
VHAI - Vocodia Partners with Leading Political Super PACs to Revolutionize Fundraising Efforts • VHAI • Sep 19, 2024 11:48 AM
Dear Cashmere Group Holding Co. AKA Swifty Global Signs Binding Letter of Intent to be Acquired by Signing Day Sports • DRCR • Sep 19, 2024 10:26 AM
HealthLynked Launches Virtual Urgent Care Through Partnership with Lyric Health. • HLYK • Sep 19, 2024 8:00 AM
Element79 Gold Corp. Appoints Kevin Arias as Advisor to the Board of Directors, Strengthening Strategic Leadership • ELMGF • Sep 18, 2024 10:29 AM
Mawson Finland Limited Further Expands the Known Mineralized Zones at Rajapalot: Palokas step-out drills 7 metres @ 9.1 g/t gold & 706 ppm cobalt • MFL • Sep 17, 2024 9:02 AM
PickleJar Announces Integration With OptCulture to Deliver Holistic Fan Experiences at Venue Point of Sale • PKLE • Sep 17, 2024 8:00 AM






