Wednesday, August 27, 2014 6:12:10 PM
8-11-14: CHI’s ImVacS 9th Annual Immunotherapies & Vaccine Summit, Boston
…12pm, Jeff Hutchins, PhD (PPHM’s VP/PreClinDEV), “PS-Targeting Antibodies Enhance Activity of Immune Checkpoint Inhibitors by Repolarizing Immunosuppressive Immune Cells Populating the Tumor Microenvironment”
• 8-11-14 PR: http://tinyurl.com/lpjy3u7 ”Preclinical Data Presented at Annual Immunotherapies and Vaccine Summit Show That the Combination of Peregrine Pharmaceuticals' PS-Targeting Antibodies and Anti-PD-1 Antibodies Significantly Reduce Breast Cancer Progression“
• DR. HUTCHINS’ PDF(31 Slides): http://tinyurl.com/oueldme (8-11-14)
MLV 8-27-2014 Update on PPHM… (George Zavoico)…








.
.
= = = = = = = = = = = = = =
PR/8-11-14: New PreClin. data on Bavi’s Immune-Stimulatory MOA presented at 9th ImVacS in Boston
http://investorshub.advfn.com/boards/read_msg.aspx?message_id=105177013
8-11-14: Preclinical Data Presented at Annual Immunotherapies and Vaccine Summit Show That the Combination of Peregrine Pharmaceuticals' PS-Targeting Antibodies and Anti-PD-1 Antibodies Significantly Reduce Breast Cancer Progression
• Combination Mediated Impressive Increase in Tumor-Fighting T-Cells and Significantly Reduce Tumor Growth by Over 78% as Compared to Anti-PD-1 Alone
• Combination Data Show Synergies That Enhance the Effectiveness of Recently Approved and Experimental Immuno-Oncology Treatments That Extend the Duration and Effectiveness of Tumor Fighting T-Cells
• Results Build on Data Seen in Studies Combining Bavituximab With Anti-CTLA-4 or Anti-PD-1 Antibody in Models of Melanoma and Colon Cancer
http://ir.peregrineinc.com/releasedetail.cfm?ReleaseID=865454
TUSTIN, 8/11/14: Peregrine Pharmaceuticals, Inc. (NASDAQ: PPHM, PPHMP), today announces preclinical data further validating the potent immune-stimulatory mechanism of its phosphatidylserine (PS)-targeting immuno-oncology platform. Results show that the combination of a PS-targeting antibody equivalent to <b<bavituximab administered with an anti-PD-1 antibody displayed statistically significant tumor growth suppression while also demonstrating a significant increase in tumor-fighting T-cells into the tumor microenvironment compared to anti-PD-1 antibody treatment alone in an immune competent animal model of breast cancer. These data will be presented today at 12:00pmET at ImVacS, the 9th Annual Immunotherapies and Vaccine Summit being held August 11-14, 2014 in Boston, Massachusetts [ http://www.imvacs.com/Immunomodulatory-Antibodies-Cancer ]. Bavituximab is currently being evaluated in second-line non-small cell lung cancer (NSCLC) as part of the SUNRISE pivotal Phase III clinical trial.
"These statistically significant results are an important extension of earlier data obtained combining our PS-targeting antibodies with other immune checkpoint inhibitors in different tumor types and our clinical experience in treating breast cancer patients," said Jeff Hutchins, Ph.D., VP of Preclinical Research at Peregrine and the study's presenter. "These data further validate that blocking the immunosuppressive effects of PS facilitate an increase in tumor-fighting T-cells, and that the combination with PD-1 then allows for a more effective anti-tumor T-cell response. We believe these studies, along with our previously released Phase II breast cancer results, warrant an expanded clinical investigation in breast cancer that would build on our ongoing immunotherapy combination clinical trial in advanced melanoma."
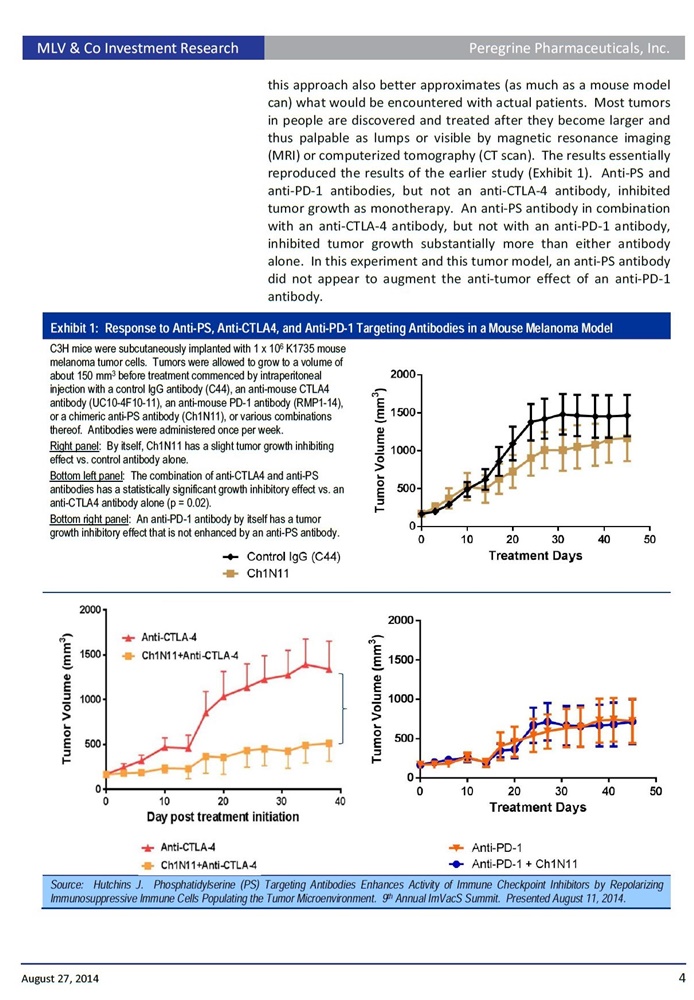
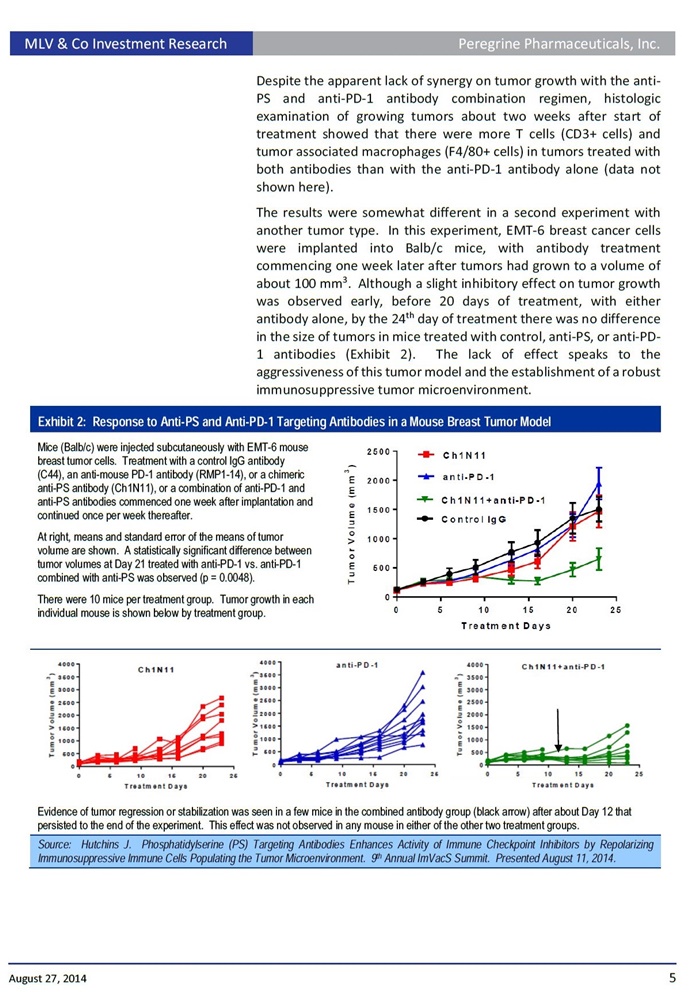
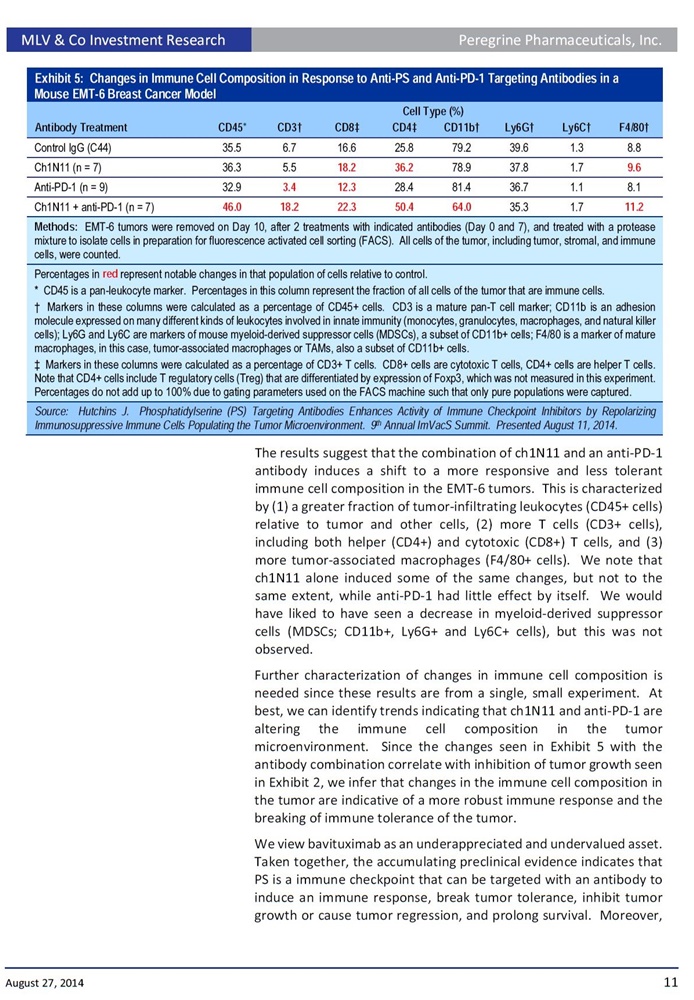
In a presentation titled: "Phosphatidylserine (PS)-Targeting Antibodies Enhance Activity of Immune Checkpoint Inhibitors by Repolarizing Immunosuppressive Immune Cells Populating the Tumor Microenvironment", Dr. Hutchins will provide an overview of the company's PS-targeting platform including preclinical data emerging from the company's immuno-oncology development program. This presentation includes new data showing that animals treated with the PS-targeting antibody ch1N11, the preclinical equivalent to bavituximab, in combination with anti-PD-1 in an EMT-6 mouse breast tumor model, significantly delayed the treatments group median tumor growth compared to anti-PD-1 alone. Specifically, following once weekly treatments of ch1N11 plus anti-PD-1, tumor growth was inhibited by 78.7% (p= 0.0048 on day 23) compared anti-PD-1 alone. In addition, 50% of the tumors treated with the combination either regressed or did not progress compared to 0% for anti-PD-1 alone. Also, the once weekly combination treatment with ch1N11 and anti-PD-1 led to a 78% and 81% increase in intratumoral CD4+ and CD8+ T cells, two key indicators that show that tumor fighting immune cells are present in the local tumor environment, compared to the single agent anti-PD-1.
"With these combination results, we are clearly seeing both a significant delay in tumor growth as a group and a decrease in the number of animals with tumor progression," said Bruce Freimark, Ph.D., Director Of Pre-Clinical Research in Oncology. "We believe these data present encouraging observations to support the expanded clinical use of tumor immunotherapy combinations using PS-targeting antibodies."
A copy of Dr. Hutchins' presentation is available on the company's website at http://www.peregrineinc.com .
[ http://www.peregrineinc.com/publications/posters-and-presentations.html
PDF (31 Slides) 8-11-14: http://www.peregrineinc.com/images/stories/pdfs/2014_imvac_summit_presentation_peregrine_pharmaceuticals.pdf ]
About Bavituximab: A Targeted Investigational Immunotherapy
Scientific research has shown that tumors evade immune detection due partly to the expression of phosphatidylserine, or PS, a highly immunosuppressive molecule exploited by tumors. Peregrine's immuno-oncology development program has developed bavituximab, an investigational PS-targeting monoclonal antibody that targets and binds to PS and blocks the immunosuppressive effects of PS while activating tumor fighting immune cells, thus enabling the immune system ability to recognize and fight cancer. Bavituximab's immune-stimulatory mechanism-of-action data is the subject of a manuscript published in the October 2013 issue of the American Association for Cancer Research (AACR) peer-reviewed journal, Cancer Immunology Research [Pub. online 8-19-13, “PS-Targeting Antibody Induces M1 Macrophage Polarization & Promotes Myeloid-Derived Suppressor Cell Differentiation”, PE-Thorpe etal http://cancerimmunolres.aacrjournals.org/content/1/4/256.abstract ]. Bavituximab is currently being evaluated in several solid tumor indications, including non-small cell lung cancer, breast cancer, liver cancer, rectal cancer and advanced melanoma. In January 2014, bavituximab received Fast Track designation by the U.S. Food and Drug Administration (FDA) for the potential treatment of second-line non-small cell lung cancer [1-6-14: http://tinyurl.com/l799ukk ].
About The SUNRISE Trial
SUNRISE is a pivotal Phase III, randomized, placebo-controlled, double-blind, multinational clinical trial evaluating the efficacy and safety of bavituximab, a novel investigational immunotherapy, plus docetaxel versus placebo plus docetaxel as a second-line treatment for patients with Stage IIIb/IV non-squamous non-small cell lung cancer (NSCLC). For additional information about the SUNRISE trial please visit http://www.sunrisetrial.com or ClinicalTrials.gov using the Identifier NCT01999673. [ http://www.clinicaltrials.gov/ct2/show/NCT01999673 ]
About Peregrine Pharmaceuticals, Inc.
Peregrine Pharmaceuticals, Inc. is a biopharmaceutical company with a pipeline of novel drug candidates in clinical trials for the treatment and diagnosis of cancer. The company's lead immunotherapy candidate, bavituximab, is in Phase III development for the treatment of second-line non-small lung cancer (the "SUNRISE trial") along with several investigator-sponsored trials evaluating other treatment combinations and additional oncology indications. The company is also advancing a molecular imaging agent, 124I-PGN650, in an exploratory clinical trial for the imaging of multiple solid tumor types. Peregrine also has in-house cGMP manufacturing capabilities through its wholly-owned subsidiary Avid Bioservices, Inc. ( http://www.avidbio.com ), which provides development and biomanufacturing services for both Peregrine and third-party customers. Additional information about Peregrine can be found at http://www.peregrineinc.com .
Safe Harbor *snip*
Contact: Christopher Keenan, Peregrine Pharmaceuticals, (800) 987-8256, info@peregrineinc.com
= = = = = = = = = = = = = = = = = = = = = = = =
Aug11–12 2014: CHI’s 2nd Annual Immunomodulatory Therapeutic Antibodies for Cancer Conf., Boston
(Part of: “ImVacS”, The 9th Annual Immunotherapies & Vaccine Summit)
“Discovery & Dev. of the Next Wave of Checkpoint Inhibitors”
http://www.imvacs.com/Immunomodulatory-Antibodies-Cancer/
CHI=Cambridge Healthtech Institute http://www.chicorporate.com - CHI is the preeminent life science network for leading researchers & business experts from top pharmaceutical, biotech, and academic organizations.
8-11-14/12pm (R&D Track):
“Phosphatidylserine (PS) Targeting Antibodies Enhances Activity of Immune Checkpoint Inhibitors by Repolarizing Immunosuppressive Immune Cells Populating the Tumor Microenvironment”
Speaker: Jeff Hutchins, PhD (VP, Pre-Clinical Dev., PPHM)
ABSTRACT:
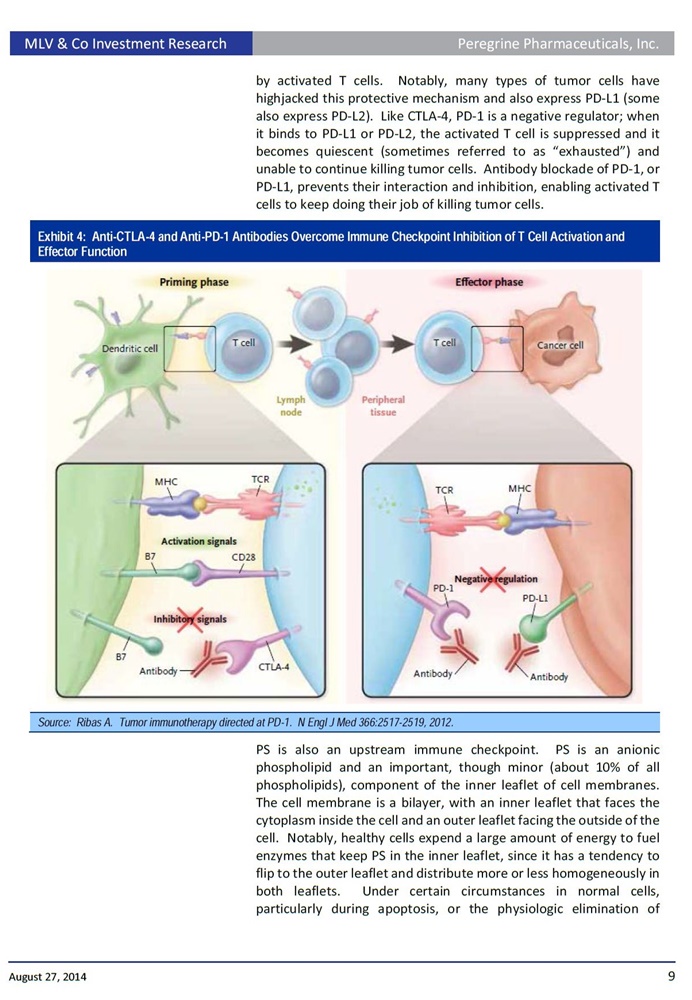
PS is a phospholipid normally residing in the inner leaflet of the plasma membrane and becomes exposed on cells of the tumor microenvironment, promoting an immunosuppressive microenvironment (MDSCs, immature dendritic cells, M2 macrophages, anti-inflammatory cytokines). Bavituximab, a PS-targeting antibody, repolarizes this microenvironment, enhancing innate and adaptive anti-tumor immunity. We demonstrate PS targeting antibodies enhance anti-tumor activity of multiple forms of standard therapy, including anti-CTLA-4 & anti-PD-1 checkpoint inhibitor antibodies without the side-effects of systemic immune activation.
= = = = = = = = = = = = = = = = = = = = = = = = = =
Bavituximab MOA & Clinical Data: http://www.peregrineinc.com/pipeline/bavituximab-oncology.html
• Bavi Publications: http://www.peregrineinc.com/publications/publications.html
• Bavi Posters & Presentations: http://www.peregrineinc.com/publications/posters-and-presentations.html
Bavi MOA: Video (3:34) added ~3-2014 http://vimeo.com/87116642 "Bavituximab: A Novel Immunotherapy Candidate Targeting an Upstream Immune Checkpoint to Fight Cancer"
Bavi MOA: Video (1:33) on Bavi’s Immunotherapeutic MOA added to Youtube on 3-27-14 https://www.youtube.com/watch?v=Esewl35JD8s
BAVI MOA 8-11-14: PPHM/VP Dr. Jeff Hutchins’ presentation at ImVacS/Boston - PR: http://tinyurl.com/lpjy3u7 ; PDF(31 Slides): http://tinyurl.com/oueldme
BAVI MOA 5-28-14: Dr. Rolf Brekken’s 47min CRI “Cancer Immunotherapy” webinar about Bavituximab as an Upstream/Global Immune Checkpoint Inhibitor – REPLAY: http://tinyurl.com/lxgftyx
. . .CRI=Cancer Research Institute (NYC – Supported by BMS): http://www.cancerresearch.org
. . .CRI launches “Answer to Cancer” (cancer immunotherapy) website http://www.theanswertocancer.org
BAVI MOA 3-25-14: Dr. Rolf Brekken’s 40min talk at NYAS Lung Cancer Symposium http://tinyurl.com/lq9stnk (45 Slides)
. . .Dr.Brekken’s talk: “Antibody-mediated Inhibition of PS - A Novel Strategy for Immune Checkpoint Blockade”
. . .The 5 speakers: Jessica Donington, Roy Herbst, Balazs Halmos, Suresh Ramalingam, Rolf Brekken
BAVI MOA: 12-2013 Bavi’s Immunotherapeutic MOA overviewed by UTSW’s Brekken/Huang in Pan European Networks Jrnl. http://tinyurl.com/lnb46pq
BAVI MOA 11-9-13: Annual SITC (WashDC) – 2 posters about Bavi’s Immunostimulatory MOA http://tinyurl.com/mjaweu5
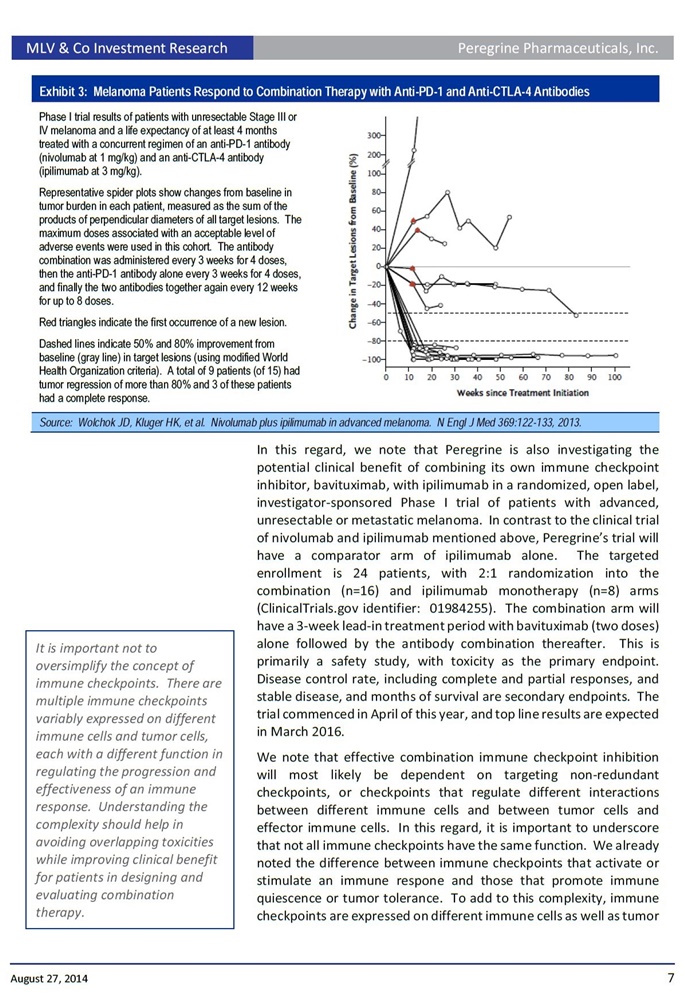
...“We are actively working towards initiating a clinical trial in the coming months to further investigate the potential synergistic effects of bavituximab and an approved [anti-CTLA-4] immunotherapy in patients with Melanoma."
10-28-13 IASLC/Sydney: “Immune Checkpoints in the Tumor Environment: Novel Targets & the Clinical Promise of Combined Immunotherapies” http://tinyurl.com/mjaweu5
…Symposium speakers: Scott J. Antonia/MD-PhD(H.Lee Moffitt CC), Dmitry I. Gabrilovich/MD-PhD(Wistar Inst), Rolf A. Brekken/PhD(UTSW), David E. Gerber/MD(UTSW)
BAVI MOA: 8-19-13 Data Supporting Bavituximab’s Immunotherapy MOA Published in “Cancer Immunology Research” (AACR) - http://tinyurl.com/mhjftka (PDF)
…“PS-Targeting Antibody Induces M1 Macrophage Polarization & Promotes Myeloid-Derived Suppressor Cell Differentiation” (Thorpe etal)
BAVI MOA: 8-13-13 PPHM/VP Dr. Jeff Hutchins’ Presentation on the Downstream Immunostimulatory Effects/Moa of PS-targeting antibodies (like Bavi) at CHI’s “Immunotherapies Congress”/Boston http://tinyurl.com/m6h2tvt
= = = = = = = = = = = = = = = = =
BAVITUXIMAB PHASE III TRIAL: see http://PeregrineTrials.com => http://www.SunriseTrial.com (Start=Dec'13 EstPrimComp=Dec’16)
A. Phase III Bavi+Doce vs. 2nd-Line NSCLC "SUNRISE" (randomized, double-blind, placebo-ctl'd, n=582)
USA Protocol: http://www.clinicaltrials.gov/ct2/show/NCT01999673 - 131 sites a/o 8-20-14 (USA/35 Aus/7 Bel/7 Fr/9 Ger/12 Greece/7 Hungary/7 Italy/2 Korea/7 Rom/6 Rus/7 Spain/16 Taiwan/9) - Growth: http://tinyurl.com/qzfo56c
...EU/EEA Registries: (pts world=582, pts/EU=345, planned EU sites=100) http://www.clinicaltrialsregister.eu/ctr-search/search?query=2013-003953-13
• GER: http://www.clinicaltrialsregister.eu/ctr-search/trial/2013-003953-13/DE (planned sites=14, pts=43)
• SPAIN: http://www.clinicaltrialsregister.eu/ctr-search/trial/2013-003953-13/ES (planned sites=16, pts=100)
• HUNGARY: http://www.clinicaltrialsregister.eu/ctr-search/trial/2013-003953-13/HU (planned sites=9, pts=28)
• GREECE: http://www.clinicaltrialsregister.eu/ctr-search/trial/2013-003953-13/GR (planned sites=9, pts=120)
...EU= European Union (29 countries – see http://www.gov.uk/eu-eea ), EEA=European Economic Area (all the EU, plus Iceland, Liechtenstein, Norway)
…Regions where sites are planned: Australia, Belgium, France, Germany, Greece, Hungary, Italy, Korea, Romania, Russia., Spain, Taiwan, Ukraine, USA
...3-2014: Eur.Med.Assoc.(EMA) appears to approve Bavi/2L-NSCLC for possible future trial in Children: http://tinyurl.com/lqsjfj2
5-31-14 ASCO’14: David Gerber/Joe Shan Poster on Ph3/SUNRISE Trial (#TPS8129) http://tinyurl.com/nv4jloo
1-6-14: FDA grants FAST TRACK status to Bavi in 2ndLine NSCLC http://tinyurl.com/l799ukk
12-30-13: Pivotal Ph.3 ‘SUNRISE’ NSCLC Trial Initiated (n=~600, sites=~100) http://tinyurl.com/kdjb9qz
5-20-13: FDA Approves Bavituximab Ph.III Design for 2L/NSCLC; 600-pt trial to begin by y/e’13 http://tinyurl.com/n3dxtm6
...S.King: “We will now focus on starting the Ph.III trial while continuing ongoing partnering discussions.”
…R.Garnick: “This was a highly collaborative effort with the FDA; this trial, when combined with Bavi’s supporting data to date, could be sufficient to support a future BLA submission."
Recent CDMO News
- Avid Bioservices to Participate in Craig-Hallum Bioprocessing Conference • GlobeNewswire Inc. • 09/12/2024 08:05:27 PM
- Form 10-Q - Quarterly report [Sections 13 or 15(d)] • Edgar (US Regulatory) • 09/09/2024 08:43:56 PM
- Form 8-K - Current report • Edgar (US Regulatory) • 09/09/2024 08:19:30 PM
- Avid Bioservices Reports Financial Results for First Quarter Ended July 31, 2024 • GlobeNewswire Inc. • 09/09/2024 08:05:31 PM
- U.S. Futures Rise Amid Inflation Report Anticipation; Oil Prices Climb on Hurricane Threat and Supply Concerns • IH Market News • 09/09/2024 10:09:14 AM
- Avid Bioservices to Report Financial Results for First Quarter of Fiscal Year 2025 After Market Close on September 9, 2024 • GlobeNewswire Inc. • 09/03/2024 08:05:20 PM
- Form 8-K - Current report • Edgar (US Regulatory) • 08/29/2024 08:30:10 PM
- Form ARS - Annual Report to Security Holders • Edgar (US Regulatory) • 08/28/2024 08:34:04 PM
- Form DEFA14A - Additional definitive proxy soliciting materials and Rule 14(a)(12) material • Edgar (US Regulatory) • 08/28/2024 08:32:18 PM
- Form DEF 14A - Other definitive proxy statements • Edgar (US Regulatory) • 08/28/2024 08:30:28 PM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 07/16/2024 11:50:20 PM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 07/16/2024 11:48:19 PM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 07/15/2024 08:40:04 PM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 07/12/2024 08:30:04 PM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 07/11/2024 09:02:39 PM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 07/11/2024 09:02:27 PM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 07/11/2024 09:01:22 PM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 07/11/2024 09:01:05 PM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 07/11/2024 09:00:54 PM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 07/11/2024 09:00:45 PM
- Form 144 - Report of proposed sale of securities • Edgar (US Regulatory) • 07/11/2024 12:23:26 AM
- Avid Bioservices Reports Financial Results for Fourth Quarter and Fiscal Year Ended April 30, 2024 • GlobeNewswire Inc. • 07/02/2024 08:05:04 PM
- Avid Bioservices to Report Financial Results for Quarter and Fiscal Year Ended April 30, 2024, After Market Close on July 2, 2024 • GlobeNewswire Inc. • 07/01/2024 11:00:21 AM
- Avid Bioservices Earns Committed Badge from EcoVadis for Sustainability Performance • GlobeNewswire Inc. • 05/23/2024 12:05:46 PM
- Avid Bioservices to Participate at Upcoming Investor Conferences • GlobeNewswire Inc. • 05/07/2024 08:05:11 PM
VHAI - Vocodia Partners with Leading Political Super PACs to Revolutionize Fundraising Efforts • VHAI • Sep 19, 2024 11:48 AM
Dear Cashmere Group Holding Co. AKA Swifty Global Signs Binding Letter of Intent to be Acquired by Signing Day Sports • DRCR • Sep 19, 2024 10:26 AM
HealthLynked Launches Virtual Urgent Care Through Partnership with Lyric Health. • HLYK • Sep 19, 2024 8:00 AM
Element79 Gold Corp. Appoints Kevin Arias as Advisor to the Board of Directors, Strengthening Strategic Leadership • ELMGF • Sep 18, 2024 10:29 AM
Mawson Finland Limited Further Expands the Known Mineralized Zones at Rajapalot: Palokas step-out drills 7 metres @ 9.1 g/t gold & 706 ppm cobalt • MFL • Sep 17, 2024 9:02 AM
PickleJar Announces Integration With OptCulture to Deliver Holistic Fan Experiences at Venue Point of Sale • PKLE • Sep 17, 2024 8:00 AM






