Register for free to join our community of investors and share your ideas. You will also get access to streaming quotes, interactive charts, trades, portfolio, live options flow and more tools.
Problem go to zero, speculating
SBFM: How long did it take you to figure-THAT-out??? (Nearly as much of a SCAM as old @TSOI & @HALB 'Health Care' firms!!! All of which belong in a SIDE SHOW, at the now defunct Ringling Bros., Barnum & Bailey CIRCUS!!!)
Anyone know if they are working on a wonder drug , speculating
Do you think sales are fake
Wonder about sales, what they are selling, ???????
Maybe revenues are fake ,MAYBE
Interesting comment
Wonder if bottom, speculating 1,000,000%
I am beginning to believe that SBFM is a scam
Interesting share structure
Terrible what happened here
Anyone know SBFMW current conversion price?
Imagine 40 billion worth with just over 1,000,000 shares, just throwing out numbers
On otc shows millions just over shares
Made off this a while back
Wonder about the cash how long it will last for
Any spectacular project they working on or company done
How many r s lately 5 ???????
How many shares outstanding these days
Price prediction
Is SBFM done R/Sing and will the price stay above $3 into 2025? Once I know this I'll be back for a few shares just for the future of Nora Pharma in 2025 and beyond.
Agreed. What is the float down to with all the reverse splits? 20 shares? Move on this will
With right news could this skyrocket, 1000% pure speculating
|
Followers
|
757
|
Posters
|
|
|
Posts (Today)
|
0
|
Posts (Total)
|
113861
|
|
Created
|
10/27/08
|
Type
|
Free
|
| Moderators | |||
SUNSHINE BIOPHARMA INC
ANTI CANCER DRUGS
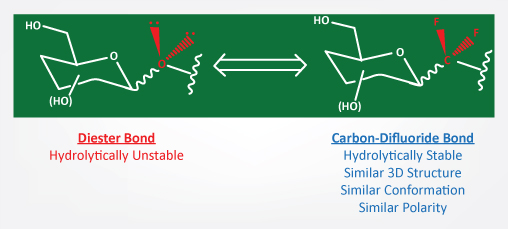
ANTI CORONA VIRUS RESEARCH








SBFM Transfer Agent
Corporate Stock Transfer
3200 Cherry Creek Dr. South
Suite 430
Denver, CO 80209
(303) 282 - 4800 p
(303) 282 - 5800 f
| Volume | |
| Day Range: | |
| Bid Price | |
| Ask Price | |
| Last Trade Time: |