Register for free to join our community of investors and share your ideas. You will also get access to streaming quotes, interactive charts, trades, portfolio, live options flow and more tools.
extrinsic55 - has there been any interest in a class action lawsuit?
Thanks
To All. Your best solution is not to go after the company but the individuals who ran and exploited the company and its investors. Once the company is completed its insolvency, then you file your lawsuits on Kempers and the mail characters. ( Class Action lawsuit ). Where you bring all MYMX investors into the suit who lost money. A good Law Firm will not charge anything until a settlement is accepted if you can prove a wrongful action was committed.
Ronald.Kempers@
AhsokaXC. What was the email you used for Kempers. I've tried MrRonaldKempers, M.RonaldKempers,RonaldKempers@mymetics.com.
None of them have gone through. Thanks
I feel there are a few folks that want to make sure they are part of the wind down if indeed there are distributions after the creditors are satisfied. I emailed Mr Kempers with the number of shares I currently hold and my interest. I don’t think it needs to be a super formal letter. But make sure you do it by Sunday midnight Swiss time which is rapidly approaching.
You can replace the name with the x’s
I asked the auditor (https://fiduciaire-bonnefous.com/) who to contact to make a claim and they said Ronald Kempers himself and gave me his email address...should I post it here?
Cher Monsieur,
Je vous laisse le soin de contacter M. Ronald Kempers.
xxxxxx.xxxxxxx@mymetics.com
Avec mes cordiales salutations.
Corinne Dumonthay
Expert-comptable diplômée, agréée ASR
Translated:
Dear Sir,
I leave it to you to contact Mr. Ronald Kempers.
xxxxxx.xxxxxxx@mymetics.com
With my cordial greetings.
Corinne Dumonthay
Qualified accountant, ASR approved
So that's who you send your requests to though I'm not really a lawyer and don't know how formal a request would be.
Thanks very much for your preliminary investigation. It seems that a 1-year clock is ticking from date of liquidation, in which the liquidator must divest of company assets and reimburse
shareholders their due. I believe that Kempers is the named liquidator…the same individual who orchestrated the massive reverse split of our shares last fall.
I have been looking everywhere for the final report of the 5-year NIH-funded HIV study that MYMX was involved in…no luck finding that yet. I do believe that a monetary accounting of the liquidation could be substantial given all the research, patents, etc. that MYMX was involved in over several decades. Worth pursuing? If so, I concur that we would need to work with a Swiss attorney if enough are willing to pursue this. Hopefully your message will get a response soon.
Can we get a head count on how many investors are interested in pursuing this?
I haven't heard anything back unfortunately...but I did find this on Swiss Liquidation
https://www.kmu.admin.ch/kmu/en/home/concrete-know-how/acquiring-selling-closing-business/end-of-the-company/voluntary-bankruptcy/closure-procedures.html
Call to creditors. This is a key stage. The liquidator must contact the Swiss Official Gazette of Commerce (SOGC) for the publication of one call to creditors (Art. 742 Code of Obligations). The board of directors carries out the call to creditors following the resolution of the General Meeting on the resolution to dissolve the company. This publication serves to inform all creditors of the winding-up of the company and to instruct them to make their claims known within the following year. Many creditors regularly consult the SOGC or mandate an economic intelligence company to do this.
Liquidation. The liquidation process starts at this stage and the process can sometimes last years. The liquidator must draw up an inventory of assets and prepare a balance sheet, which includes debt claims arising as a result of publication of calls to creditors in the SOGC. It must complete current business, execute the company’s commitments and liquidate the assets. If the assets no longer cover the debts, it informs the court, which declares bankruptcy. If any assets remain once debts are paid, the liquidator distributes them among the shareholders in proportion to their payments, also taking into account the preferential rights attached to their shares in the company. With respect to a cooperative company, when the articles of association do not provide for an allocation of surplus assets among shareholders, this surplus must be allocated for cooperative purposes or for other purposes in the public interest.
Deletion from the trade register. One year at the earliest after publication of the call to creditors, the liquidator may ask the trade register to delete the company, provided liquidation is complete.
I messaged a Swiss lawyer. Will let you know what I hear back. It shouldn’t be so hard to tell shareholders even if a private company what is happening.
It seems we only have until May 19 to file claims with MYMX. If anything is in the works please post so others can join/support the effort. I did try to track down the final report of the 5 year NIH-funded grant, but have not yet seen it. Does anyone have
access to a report of findings that Dr. Ruth Ruprecht may have published this month? Haven’t found that either.
If anyone is in contact with a lawyer or knows someone, I would certainly contribute my shares to the 10%. I thought extrinsic55 had a lawyer.
I'd like to know if what they're doing is legal without contacting other shareholders.
In my opinion it's all over. And I have no clue what would be possible. A specialized lawyer could tell you - you should remember that the legal form of the company is not simple and the company is controlled by a few insiders holidng the majority of shares. As far as I understand it, they can decide almost what they want.
But if you really want, you could try:
1. Request from the company information about the next general meeting.
2. There all minority shareholders have the right to request information about company matters or call for a special audit.
3. From German Wikipedia, automatically translated: "If the AGM rejects the latter, a total of at least 10 percent of the share capital or alternatively CHF 2 million nominal value can request the competent court to order an independent special audit."
(If you do this and if it's necessary I would contribute my percentage that these 10% may be reached.)
But I don't think this would lead to any meaningful result. The company is full of debt. There is nothing to distribute. Except if the NIH/HIV project was successful.
I don't know, but it could be possible that this project was successful and the company uses the liquidatin process to sell all the intellectual property. Then perhaps we could see some money. But firstly they don't tell us anything and secondly I don't believe so. If I am right, they could simply take all intellectual property out of the company in exchange for debt and close the company. Then they would not need to pay anything to other shareholders. Perhaps that's even fully legal. But again: I have no clue, I am not a lawyer.
I just spent 24 hours trying to get access to official documents related to the MYMETICS SA liquidation process and how we creditors can file claims. It has been hard, as all documents/publications are in French. I requested that the publishing journal send a version in English (which their web site says they do), but they responded negatively. I don’t speak or read French, but perhaps others here do.
Relevant documents are published in the Swiss Official Gazette of Commerce (SOGC), file #CHE-113.618.351. What I gleaned from this is the following:
New Address: Mymetics SA
Route de la Corniche 4
1066 EpalingesSwitzerland
There was a change of exec officers /reps on 1/3, 2024
SOGC daily register #86 SOGC publ #1005928290
another change of exec officers/reps on 1/31/2024
SOGC daily register #2447 SOGC publ #1005952896
liquidation and change of exec officers/reps on 4/10 2024
SOGC daily register #7598 SOGC publ #1006009160
I believe claims must be filed by by May 19, 2024. How do we do that? Will appreciate any help regarding this.
I agree. How can we pursue getting proper information on the liquidation and investor remuneration? Seems there is only a brief window of 2 weeks or so to submit a claim?
I don't know.
But I know that the company did not inform me/us sufficiently.
Fire sale for cheap I guess? What a huge disappointment.
They liquidate:
https://shab.ch/#!/search/publications/detail/ea07e4c0-e37b-4217-b4a0-ad4445486451
Kempers is liquidator.
I would expect him to inform us if there is something to liquidate. And: What's about NIH?
Should turn Alt Reporting any day now.
Thanks siar. It says, "Mymetics has provided its commitments
to this project by delivering the vaccines, which are tested in non-human primates."
The contract ends April 30, 2024. I wonder if the results will be listed on the NIH website or will MYMX release them. I guess it's possible we may not know for some time. Who owns the rights to the vaccine NIH or MYMX. If NIH is happy with the results, what do you think would be the next step. Would it become part of their portfolio? I don't know how MYMX would be able to continue working on a vaccine unless they worked with a larger company in a possible buy out.
Just checked it. It does not say, the results will be released in April, it says testing or the end of the project is April 30, 2024.
You will find that and details about it in the 10-Q from 11/20/2023 at the heading "NIH", e.g. "The Project with ULL under the NIH grant for our HIV vaccine is in its final year that will end on April 30, 2024. Mymetics has provided its commitments to this project by delivering the vaccines, which are tested in non-human primates."
I think, release could be before that date, after or even never, if Mymetics just sells that all.
By the way: Still some people seem to believe that Mymetics is not yet done completely. I always see bids for almost buying all outstanding shares (for nearly nothing).
siar, where did you read the HIV were going to be released in April. I don't recall seeing that. That could possibly provide some good news. Thanks.
I hope that’s the case. It’s frustrating they couldn’t make a deal earlier with so many patents and tech. But we just have to wait it out now.
Thanks for posting that, AhsokaXC. A small group of us public shareholders left … we remain in patient waiting mode. It’s very hard to believe that MYMX is not “in the running” for some kind of merger/buyout. Their portfolio and patents are just way too valuable to throw away. Haven’t seen any selling by insiders, and they know far more than we do.
As Siar notes, the HIV study results next month may prove a catalyst to MYMX news.
Yes, it was another company.
Now I am waiting for the HIV results, in April, if I remember correctly.
Actually a company has to wait 90 days after filing the Form 15-12G before the ticker is deregistered. They can't speed that up.
I don't think the ticker would go to Expert Market anyway until OTCmarkets.com realized they didn't file an annual report. That would be March 31st or right afterwards, coincidentally also 90 days.
I never expected a complete wipeout here. How can all their tech and patents be totally worthless? It seems more like an inside move to feather someone's nest.
I'll be filing a SEC complaint like most here but it's difficult to press the case unless you can point to egregious and blatant fraud. I don't expect much to come of it,
Happy New Year and Best to all
So today is the day MYMX shut down as a public company. How can remaining shareholders communicate among ourselves; is there a chat room available to us?
assume you found it: MYMXD
MYMX one for 2,000 reverse split:
https://otce.finra.org/otce/dailyList?viewType=Symbol%2FName%20Changes
By it's company name or just put it in your favourite boards list.
Then how would one access it?
I guess they are offering to buy stock. So if you can get a 100% return immediately maybe that’s the play? But I think there’s more going on behind the scenes.
I think this message board will remain available, but surely not accessable by means of the ticker symbol.
Now 4.3 million on bid.
Agree with your sentiments. Will this site remain available for us to communicate as MYMX goes private?
New 8-K.
"[...] to effectuate the Reverse Stock Split, and such amendment was effective as of 12:01 a.m. on December 28, 2023."
While trading?
"On or about January 2, 2023, the Company is filing a Form 15 [...]"
I am disappointed with the performance of Mr. Kempers, so now I could be a bit sarcastic and praise that he is once ahead of all others.
And still I would like to know who is willing to buy 3.7 million shares a few days before going private.
Merry Christmas everyone. I hope you all have a wonderful day and also hope we get good news soon.
I think the creditors have first right of access to the assets.
And it seems to be clear, that negotiations are going on, or are just finished. For cost reduction the company has to go private today or next week. This is already a tight timeframe.
I am curious about the outcome of Mr. Kempers "gamble". He seems to have updated, better to say, cleared and hided a bit of his linkedin account.
In a few days we will see whether we could roughly get back our investment or loose everything - I think there's almost no other option.
MYMX’s two major creditors - Round Enterprises LTD and Eardley Holding, AG - have clauses for over a decade stating that their loans are “secured against all property of the Company”. Does that relate here?
Not sure siar. It says the assets are held by a secured creditor. I haven't seen any filing saying MYMX assets are being held by a creditor.
It seems the last 5 filings are just withdrawing stock options from years ago. I read a notice of effectiveness was when they want to sell securities, but I guess it's to sell or withdraw. Is that correct?
Just read this and wondering if there could be a relation to MYMX
https://www.otcmarkets.com/stock/BVAXF/news/BioVaxys-in-Non-Binding-Discussions-for-Major-Immunotherapeutics-Technology-Acquisition?id=424591
Never under estimate a shareholder
|
Followers
|
86
|
Posters
|
|
|
Posts (Today)
|
0
|
Posts (Total)
|
27660
|
|
Created
|
09/25/02
|
Type
|
Free
|
| Moderators | |||

http://www.mymetics.com/
COMPANY OVERVIEW
https://www.mymetics.com/files/8116/2081/0445/May21-Mymetics_Overview.pdf
Mymetics Corporation is US registered biotechnology company with its main offices in Switzerland and the Netherlands.
Focused on developing next generation preventative vaccines for infectious diseases.
Mymetics core technology and expertise are in the use of virosomes,
lipid-based carriers containing functional fusion viral proteins in combination with rationally designed antigens and membrane proteins.
Objective: "Build small / medium size innovative R&D virosome vaccine company with strong
partnerships, Phase II – III clinical vaccine pipeline and have optionality for M&A or sale."
Current Share Structure:
Outstanding Shares: 303.7 million (as of 01/18/2019 unchanged since 2014)
Floating Shares: 79.8 million* (a/o 01/18/19)
(https://www.otcmarkets.com/stock/MYMX/profile)
Rationale and Impact of MACIVIVA
With few exceptions, commercialized vaccines are generally delivered by injection through the intramuscular or subcutaneous route.
Vaccines contain immunogens classically found within a large variety of biological compounds such as peptides, proteins, glycoproteins and sometimes carbohydrates and lipids.
These immunogens may trigger the immune system for producing antibodies and/or cytotoxic T cells for preventing the pathogen transmission or blocking and/or slowing down the disease progression.
However, these vaccines generally exist as liquid formulation that are inherently prone to physical and/or chemical modifications. The cold chain storage is still fundamental for preserving the
bioactivity of most liquid and freeze-dried vaccines. For reconstituted freeze dried vaccines, they harbor important instability and must be used within hours and kept refrigerated. Vaccine degradation generally takes place
during shipment and/or storage of liquid or lyophilized products, which may affect the immunological properties of the immunogens, with unwanted immune responses or insufficient immune protection.
There is growing evidence that solid dosage formats (e.g. powder form) for vaccines may offer several advantages over the liquid formulations, such as the prevention of molecular motion and shear-induced degradation,
and slowing down modifications and degradation reactions involving water and oxygen radicals, resulting in improved stability, enhanced shelf-life of vaccines and greatly simplified logistics.
MYMETICS BV
Expertise: R&D on virosome formulations
Project responsability: Investigating and compiling the results about the physical and biochemical properties of the virosome-based vaccines obtained by spray-drying and lyophilization.
MYMETICS SA
Expertise : Non-GMP and GMP virosome production, clinical development
Project responsability: Excipient selection for liquid virosomes, supervising the non-GMP and GMP manufacturing of the liquid virosomes and development of analytical methods.
UPPERTON LIMITED
Expertise: Non-GMP and GMP Spray drying
Project responsability: Identification of excipients and experimental conditions suitable for virosome spray drying, production of non-GMP and GMP powder forms for nasal and oral delivery.
CATALENT U.K. SWINDON ZYDIS LIMITED
Expertise: Zydis technology for fast-dissolving tablet, world leader in drug formulation and distribution
Project responsability: Identification of excipients and experimental conditions suitable for virosome lyophylization, according to the Zydis technology, non-GMP and GMP tablets for sublingual delivery.
CHIMERA BIOTEC GMBH
Expertise: Ultra sensitive immunoassays development and bioanalysis based on Imperacer® (Immuno-PCR) technology.
Project responsability: Immunogenicity study in animals with spray-dried and lyophilized virosomes. Imperacer® immunoassay development and evaluation of the vaccine-induced antibody response.
BACHEM AG
Expertise: R&D, non-GMP and GMP manufacturing of API, world supplier
Project responsability: Process Development and manufacture of peptide P1, GMP-grade, including development and validation of analytical methods.

Vaccines are poorly accessible in developing countries
Vaccines require cold-chain storage and are often delivered by injection, which is undesirable, less safe and more expensive to administer.
Developing thermostable solid form vaccines through non-invasive routes may represent a long-term global solution to the vaccination challenge (Amorij, 2008).
Virosomes are an efficient vaccine delivery system
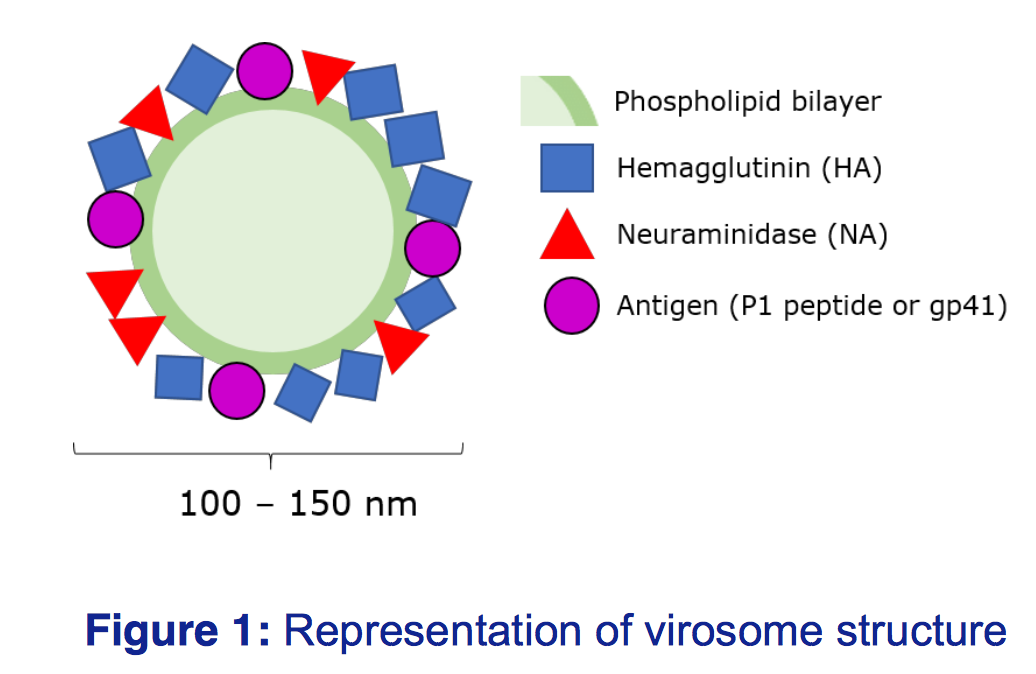
Virosomes are spherical, unilamellar lipid-based carriers, intercalated with functional glycoproteins to reflect the natural virus, however the lack of viral RNA means there is no risk of infection
(Figure 1). Virosomes can be tagged with different antigens and adjuvants, meaning they can be tailored to target different viruses, and offer increased immunogenicity over inactivated viruses.
Currently, virosomal influenza vaccines are only available in liquid form (Amorij, 2008).
Spray drying can produce dry powders for a range of dosage forms, including inhaled or nasal drug delivery.
A dry powder is formed when a liquid feed solution or suspension is atomised using a spray nozzle, and rapidly dried using hot air. However, while the drying process is gentle due to evaporative cooling,
there is still the potential to stress and inactivate vaccine components. It has been found that subunit and live-attenuated vaccines (and other delicate molecules such as proteins)
can be protected during processing b by incorporating them in an amorphous sugar matrix, which also offers longer term stability during storage (Kanojia, 2016).
A method has been developed to produce a powder form of virosome based influenza vaccine using spray-drying.
Formulations have been optimised for oral and nasal delivery.



Virosomal technology is approved by the FDA for use in humans, and has a high safety profile
Virosomes are biodegradable, biocompatible, and non-toxic12
No disease-transmission risk
No autoimmunogenity or anaphylaxis10
Broadly applicable with almost all important drugs (anticancer drugs, proteins, peptides, nucleic acids, antibiotics, fungicides)
Enables drug delivery into the cytoplasm of target cell
Promotes fusion activity in the endolysosomal pathway
Protects drugs against degradation
Intellectual Property
| Volume | |
| Day Range: | |
| Bid Price | |
| Ask Price | |
| Last Trade Time: |
