Register for free to join our community of investors and share your ideas. You will also get access to streaming quotes, interactive charts, trades, portfolio, live options flow and more tools.
Immune Therapeutics Inc., IMUN, changed to Biostax Corp., BTAX:
https://otce.finra.org/otce/dailyList?viewType=Symbol%2FName%20Changes
Bankruptcy? It has been just a shell company for a long time now.
STAB did close to $75m in dollar volume. IMUN barely got $125k. Less than a half a percent. And the float is a tenth of the size.
If this got even a half a percent of the volume it could have done 500% on the day easy.
at the end ofthe day. STAB was up 67,5% and IMUN 53.6%
Absolutely pathetic that this ticker barely moved today with their counterpart up over 130%
Statera Biopharma (Nasdaq: STAB) (the "Company"), a leading biopharmaceutical company creating next-generation immune therapies that focus on immune restoration and homeostasis, announced today that the Company entered into a non-binding term sheet with respect to a strategic agreement with Immune Therapeutics, Inc. (OTC-PINK: IMUN), a drug development and commercialization company, to sell Statera's rights to naltrexone and met-enkephalin. The transaction is contingent upon negotiation of a definitive agreement and satisfaction of a number of closing conditions, including a contingency on Immune Therapeutics financing.
"The agreement with Immune will enable us to strengthen our financial position with a transaction that has the potential to produce significant non-dilutive cashflow to fund our other programs. For instance, Statera is beginning to chart a new course in the immunotherapy field by pursuing molecules that act on Toll-like Receptor pathways similar to Naltrexone," said Michael K. Handley, President and Chief Executive Officer of Statera Biopharma. "It also will help advance our new product candidates for treatment of a variety of immune-related diseases that have no cure."
Under the anticipated terms of the agreement, Statera will receive an initial $2 million upfront payment and 5% of the issued and outstanding stock of Immune Therapeutics. Additionally, Statera will receive payments for achievement of revenue-based milestones, new indications and royalties, Crohn's disease and COVID-19 indications and regulatory approvals, as well as any other payments from Immune Therapeutics in exchange for Statera's rights to any product containing low-dose naltrexone as an active ingredient. Potential indication payments will include asthma, multiple sclerosis, HIV and chemotherapy. In aggregate, this transaction has the ability to generate over $400 million in non-dilutive payments to Statera.
"Statera's naltrexone assets will be a great addition to our immune-modulation products and immunotherapy technologies," said Kevin Phelps, CEO, Immune Therapeutics. "We believe that they can significantly enhance our ability to combat chronic, lifeâ?threatening diseases and bring hope to millions of patients and their families."
#DDAmanda Chart on: $IMUN up 100% :
You can scan for these before they run.

#DDAmanda Promo Code: dsh888
What the Fact (Factor) Column is:
The Factor is a proprietary indicator used for scanning in #DDAmanda.
It's defined as Today's $Traded divided by the average daily $Traded (20 day avg).
SO, if a stock has say a 10 Factor that day, it means she traded 10 Times the $ she normally trades.
That's significant, and many times indicates that a run in the stock is coming.

Huge long pump in-progress. Bag_holders_take_note.
https://twitter.com/realwillmeade/status/1472955487767478278
$BFRI, $CRTX, $IMUN, $LGVN, and more make up our list of most shorted stocks for Friday! https://t.co/ssLJgBzk4k
— InvestorPlace (@InvestorPlace) December 17, 2021
They also have debt and no business plan as of right now so that could be factoring into the low stock price. The company is just a shell with an investment. What is the plan?
Read the latest quarterly report from imun. Here is part of what was stated regarding stab shares:
On September 28, 2021, Cytocom announced the completion of its merger with Cleveland BioLabs, Inc. (“CBLI”) which resulted in the Company’s receipt of 1,150,000 common shares of CBLI, reflecting the Company’s retained minority interest in Cytocom. The merger was completed in July 2021. Subsequent to the merger, CBLI adopted a new corporate name, Stratera BioPharma, Inc., with the ticker symbol “STAB” effective September 1, 2021. Cytocom emerged as a publicly traded entity following the merger with CBLI.
Are you saying that IMUN owns about 3.5% of STAB? How do you come up with that percentage?
Just how bad and incompetent does the investment world think imun management really is? Well, imun current holdings of stab stock is valued at more than $9/share and yet the stock trades below $3.
The complete ignorance and deceit that was heavily promoted about this stock is disgraceful and despicable.
Yep...14.00 on 5/6/21 post MASSIVE RS. Now look.....
Hell..about time to pull another one!!
Was there really a prediction that imun would go to $160? What a joke.
Just a matter of time before this one goes back to being a penny.
Uplift? What a joke.
Cytocom, Inc. Reports Second Quarter 2021 Financial Results
https://www.cytocom.com/investors/
Cytocom, Inc. and La Jolla Institute for Immunology Announce
Five-Year Research Alliance Agreement
Cytocom to fund research in cancer, autoimmune and inflammatory conditions, and infectious diseases and applications to clinical-stage therapies
Research to accelerate immune-modulating drug development, leveraging world-class research infrastructure and Cytocom’s proprietary AIMS™ discovery platform
FORT COLLINS, Colo., August 12, 2021 /PRNewswire/ -- Cytocom, Inc. (NASDAQ: CBLI), a leading biopharmaceutical company creating next-generation immune therapies that focus on immune restoration and homeostasis, today announced a collaboration agreement to fund research and laboratory facilities at the La Jolla Institute for Immunology (LJI), a not-for-profit academic institution and a world leader in immunology research. The agreement is directed to research that will support the development of potential new immune-modulating agents targeting toll-like receptors for the treatment of cancer, infectious, autoimmune and chronic inflammatory diseases. The research will harness Cytocom’s proprietary drug discovery and development platform technology.
“An alliance with an academic institution the caliber of the La Jolla Institute marks a major achievement for Cytocom and our mission to advance best-of-class immune-modulating therapies that restore immune homeostasis,” stated Michael K. Handley, President and CEO of Cytocom. “With the completion of the merger between Cleveland BioLabs and Cytocom, we have built a robust pipeline of immune-modulating treatments targeting neutropenia and anemia, emergent viruses, cancer, and autoimmune diseases. Working with the La Jolla Institute, we will deeply explore the mechanisms that we believe will drive next-generation therapeutic development with our AIMS™ technology and bring hope to patients and their families battling serious medical conditions.”
Under the terms of the research agreement, the La Jolla Institute may select up to four laboratories to participate in research. Cytocom will provide research funding to these laboratories for projects of mutual interest or for research projects commissioned by Cytocom that explore immune modulation and the action of therapeutics on target toll-like receptors. Toll-like receptors are central to an immune response, connecting innate and adaptive immune compartments, and thus key to fighting disease as well as restoring immune homeostasis. In addition to the research funding for the selected projects, Cytocom will pay to the La Jolla Institute $350,000 per year for each selected laboratory, for a total annual discretionary funding contribution of up to $1.4 million, in addition to the research funding itself. Cytocom will also provide researchers at the La Jolla Institute with samples and materials. In return, Cytocom will have a first option to negotiate a license to new discoveries by the La Jolla Institute that arise from the research projects of common interest funded by Cytocom, however Cytocom will own any new discoveries that arise from research projects of interest to Cytocom that have been commissioned to the La Jolla Institute as “work for hire.”
“We are excited to work with Cytocom to interrogate the mechanisms of immune modulation and human immunity modulated through toll-like receptors,” stated La Jolla Institute President and Chief Scientific Officer Mitchell Kronenberg, Ph.D. “Collaborations between academic research organizations and the biopharmaceutical industry play a key role in advancing science and informing drug development to the benefit everyone involved, from discovery-oriented scientists to therapeutic-oriented companies. Such collaborations are an important element of our history and our mission looking to the future. Most importantly, the combined efforts provide hope to the millions of people worldwide who deserve immunotherapies that deliver a healthy life without disease.”
About the La Jolla Institute for Immunology
Founded in 1988, La Jolla Institute for Immunology is a nonprofit, independent biomedical research institute focused on improving human health through increased understanding of the immune system. Its scientists carry out research seeking new knowledge leading to the prevention of disease through vaccines and the treatment and cure of infectious diseases, cancer, inflammatory, and autoimmune diseases such as rheumatoid arthritis, type 1 (juvenile) diabetes, Crohn’s disease and asthma. To learn more about the Institute’s work, visit www.lji.org.
About Cytocom
Cytocom, Inc. is a clinical-stage biopharmaceutical company developing novel immunotherapies targeting autoimmune, neutropenia/anemia, emerging viruses and cancers based on a proprietary platform designed to rebalance the body’s immune system and restore homeostasis. The company also has one of the largest platforms of toll-like immune receptors (TLR4, TLR5 and TLR9) in the biopharmaceutical industry, addressing conditions such as radiation sickness and cancer treatment side effects. Cytocom is developing therapies designed to elicit directly within patients a robust and durable response of antigen-specific killer T-cells and antibodies, thereby activating essential immune defenses against autoimmune, inflammatory, infectious diseases, and cancers. Specifically, Cytocom has several clinical-stage development programs for Crohn’s disease, hematology, pancreatic cancer, and COVID-19 in addition to expansion to fibromyalgia and multiple sclerosis. To learn more about Cytocom, Inc., please visit www.cytocom.com.
Forward Looking Statements:
This press release contains forward-looking statements that involve risks and uncertainties. All statements other than statements of current or historical fact contained in this press release, including statements regarding the expected clinical development timeline for our product candidates, our future financial position, business strategy, new products, budgets, liquidity, cash flows, projected costs, regulatory approvals, the impact of any laws or regulations applicable to the company, and plans and objections of management for future operations, are forward-looking statements. The words "anticipate," "believe," "continue," "should," "estimate," "expect," "intend," "may," "plan," "project," "will," and similar expressions, as they relate to us, are intended to identify forward-looking statements. We have based these forward-looking statements on the current expectations about future events held by management. While we believe these expectations are reasonable, such forward-looking statements are inherently subject to risks and uncertainties, many of which are beyond our control. The company’s actual future results may differ materially from those discussed here for various reasons. The company discusses many of these risks under the heading "Risk Factors" in the proxy statement/prospectus filed with the SEC on June 10, 2021, as updated by the company’s other filings with the SEC. Factors that may cause such differences include, but are not limited to, the outcome of any legal proceedings that have been or may be instituted against the company related to the merger between legacy Cytocom and Cleveland BioLabs; unexpected costs, charges or expenses resulting from the merger; our need for additional financing to meet our business objectives; our history of operating losses; our ability to successfully develop, obtain regulatory approval for, and commercialize our products in a timely manner; our plans to research, develop and commercialize our product candidates; our ability to attract collaborators with development, regulatory and commercialization expertise; our plans and expectations with respect to future clinical trials and commercial scale-up activities; our reliance on third-party manufacturers of our product candidates; the size and growth potential of the markets for our product candidates, and our ability to serve those markets; the rate and degree of market acceptance of our product candidates; regulatory requirements and developments in the United States, the European Union and foreign countries; the performance of our third-party suppliers and manufacturers; the success of competing therapies that are or may become available; our ability to attract and retain key scientific or management personnel; our historical reliance on government funding for a significant portion of our operating costs and expenses; government contracting processes and requirements; the exercise of significant influence over our company by our largest individual stockholder; the impact of the novel coronavirus ("COVID-19") pandemic on our business, operations and clinical development; the geopolitical relationship between the United States and the Russian Federation as well as general business, legal, financial and other conditions within the Russian Federation; our ability to obtain and maintain intellectual property protection for our product candidates; our potential vulnerability to cybersecurity breaches; and other factors discussed in our SEC filings, including our Annual Report on Form 10-K for the year ended December 31, 2020 and the risk factors discussed under the heading “Risk Factors” in the proxy statement/prospectus the company filed in connection with the merger on June 10, 2021.
Given these uncertainties, you should not place undue reliance on these forward-looking statements. The forward-looking statements included in this press release are made only as of the date hereof. We do not undertake any obligation to update any such statements or to publicly announce the results of any revisions to any of such statements to reflect future events or developments.
Contacts:
Cytocom, Inc.
Nichol Ochsner
Senior V.P. Investor Relations and Corporate Communications
(732) 754-2545
nichol.ochsner@cytocom.com
Tiberend Strategic Advisors, Inc.
Maureen McEnroe, CFA (Investors)
(212) 375-2664
mmcenroe@tiberend.com
Johanna Bennett (Media)
(212) 375-2686
jbennett@tiberend.com
$2.55, what a sad joke played on imun shareholders. Ceo should be locked up after the horrible reverse split. What happened to the value of the cyto shaes owned by imun? Scam Citicus.
2021-08-11 07:30:00 ET (1 hour ago)
Cytocom, Inc. Announces Participation at Liberty University's Empowering the Kingdom Through Business Conference
FORT COLLINS, Colo., Aug. 11, 2021 /PRNewswire/ -- Cytocom, Inc. (NASDAQ: CBLI), a leading biopharmaceutical company creating next-generation immune therapies that focus on immune restoration and homeostasis, today announced that the company will participate at the Empowering the Kingdom through Business Conference hosted by the Liberty University School of Business on August 10-12, 2021. Cytocom President and CEO, Mike K. Handley, will participate live as a featured panelist in the Healthcare Breakout session moderated by Dean Joseph Johnson on August 11, 2021.
About Cytocom
Cytocom, Inc. is a clinical-stage biopharmaceutical company developing novel immunotherapies targeting autoimmune, neutropenia/anemia, emerging viruses and cancers based on a proprietary platform designed to rebalance the body's immune system and restore homeostasis. The company also has one of the largest platforms of toll-like immune receptors (TLR4, TLR5 and TLR9) in the biopharmaceutical industry, addressing conditions such as radiation sickness and cancer treatment side effects. Cytocom is developing therapies designed to elicit directly within patients a robust and durable response of antigen-specific killer T-cells and antibodies, thereby activating essential immune defenses against autoimmune, inflammatory, infectious diseases, and cancers. Specifically, Cytocom has several clinical-stage development programs for Crohn's disease, pancreatic cancer, COVID-19 in addition to expansion to fibromyalgia and multiple sclerosis. To learn more about Cytocom, Inc., please visit www.cytocom.com.
Forward Looking Statements:
This press release contains forward-looking statements that involve risks and uncertainties. All statements other than statements of current or historical fact contained in this press release, including statements regarding our future financial position, business strategy, new products, budgets, liquidity, cash flows, projected costs, regulatory approvals, the impact of any laws or regulations applicable to the company, and plans and objectives of management for future operations, are forward-looking statements. The words "anticipate," "believe," "continue," "should," "estimate," "expect," "intend," "may," "plan," "project," "will," and similar expressions, as they relate to us, are intended to identify forward-looking statements. We have based these forward-looking statements on the current expectations about future events held by management. While we believe these expectations are reasonable, such forward-looking statements are inherently subject to risks and uncertainties, many of which are beyond our control. The company's actual future results may differ materially from those discussed here for various reasons. The company discusses many of these risks under the heading "Risk Factors" in the proxy statement/prospectus filed with the SEC on June 10, 2021, as updated by the company's other filings with the SEC. Factors that may cause such differences include, but are not limited to, the outcome of any legal proceedings that have been or may be instituted against the company related to the merger between Cytocom and Cleveland BioLabs; unexpected costs, charges or expenses resulting from the merger; our need for additional financing to meet our business objectives; our history of operating losses; our ability to successfully develop, obtain regulatory approval for, and commercialize our products in a timely manner; our plans to research, develop and commercialize our product candidates; our ability to attract collaborators with development, regulatory and commercialization expertise; our plans and expectations with respect to future clinical trials and commercial scale-up activities; our reliance on third-party manufacturers of our product candidates; the size and growth potential of the markets for our product candidates, and our ability to serve those markets; the rate and degree of market acceptance of our product candidates; regulatory requirements and developments in the United States, the European Union and foreign countries; the performance of our third-party suppliers and manufacturers; the success of competing therapies that are or may become available; our ability to attract and retain key scientific or management personnel; our historical reliance on government funding for a significant portion of our operating costs and expenses; government contracting processes and requirements; the exercise of significant influence over our company by our largest individual stockholder; the impact of the novel coronavirus ("COVID-19") pandemic on our business, operations and clinical development; the geopolitical relationship between the United States and the Russian Federation as well as general business, legal, financial and other conditions within the Russian Federation; our ability to obtain and maintain intellectual property protection for our product candidates; our potential vulnerability to cybersecurity breaches; and other factors discussed in our SEC filings, including our Annual Report on Form 10-K for the year ended December 31, 2020 and the risk factors discussed under the heading "Risk Factors" in the proxy statement/prospectus the company filed in connection with the merger on June 10, 2021.
Given these uncertainties, you should not place undue reliance on these forward-looking statements. The forward-looking statements included in this press release are made only as of the date hereof. We do not undertake any obligation to update any such statements or to publicly announce the results of any revisions to any of such statements to reflect future events or developments.
Contacts:
Cytocom, Inc.
Nichol Ochsner
Senior V.P. Investor Relations and Corporate Communications
(732) 754-2545
[email hidden - please find in source]
Tiberend Strategic Advisors, Inc.
Maureen McEnroe, CFA (Investors)
(212) 375-2664
[email hidden - please find in source]
Johanna Bennett (Media)
(212) 375-2686
[email hidden - please find in source]
SOURCE Cytocom Inc.
Cytocom, Inc. to Report Second Quarter 2021 Financial Results
Executive Management to Host Conference Call on Monday, August 16th at 8:30 a.m. ET
FORT COLLINS, Colo., August 10, 2021 /PRNewswire/ -- Cytocom, Inc. (NASDAQ: CBLI), a leading biopharmaceutical company creating next-generation immune therapies that focus on immune restoration and homeostasis, today announced that the Company will host a conference call and live audio webcast on Monday, August 16, 2021, at 8:30 a.m. ET, to discuss its corporate and financial results for the second quarter 2021.
Conference Call & Audio Webcast Details
Date
Monday, August 16, 2021
Time
8:30 a.m. ET
Telephone Access: U.S. and Canada
833-317-6003
Telephone Access: International
412-317-6061
Access Code for All Callers
3735775
Live Audio Webcast
https://www.cytocom.com/investors/
See “Investors & Media” Section
A live webcast and audio archive for the event may be accessed from the “Investors” section of the Cytocom website at https://www.cytocom.com/investors/. Please connect to the website prior to the start of the presentation to ensure adequate time for any software downloads that may be necessary to listen to the webcast. A replay of the webcast will be archived on the website for 90 days beginning at approximately 10:00 a.m. ET, on August 16, 2021.
About Cytocom
Cytocom, Inc. is a clinical-stage biopharmaceutical company developing novel immunotherapies targeting autoimmune, neutropenia/anemia, emerging viruses and cancers based on a proprietary platform designed to rebalance the body’s immune system and restore homeostasis. The company also has one of the largest platforms of toll-like immune receptors (TLR4, TLR5 and TLR9) in the biopharmaceutical industry, addressing conditions such as radiation sickness and cancer treatment side effects. Cytocom is developing therapies designed to elicit directly within patients a robust and durable response of antigen-specific killer T-cells and antibodies, thereby activating essential immune defenses against autoimmune, inflammatory, infectious diseases, and cancers. Specifically, Cytocom has several clinical-stage development programs for Crohn’s disease, pancreatic cancer, COVID-19 in addition to expansion to fibromyalgia and multiple sclerosis. To learn more about Cytocom, Inc., please visit www.cytocom.com.
Forward Looking Statements:
This press release contains forward-looking statements that involve risks and uncertainties. All statements other than statements of current or historical fact contained in this press release, including statements regarding our future financial position, business strategy, new products, budgets, liquidity, cash flows, projected costs, regulatory approvals, the impact of any laws or regulations applicable to the company, and plans and objectives of management for future operations, are forward-looking statements. The words "anticipate," "believe," "continue," "should," "estimate," "expect," "intend," "may," "plan," "project," "will," and similar expressions, as they relate to us, are intended to identify forward-looking statements. We have based these forward-looking statements on the current expectations about future events held by management. While we believe these expectations are reasonable, such forward-looking statements are inherently subject to risks and uncertainties, many of which are beyond our control. The company’s actual future results may differ materially from those discussed here for various reasons. The company discusses many of these risks under the heading "Risk Factors" in the proxy statement/prospectus filed with the SEC on June 10, 2021, as updated by the company’s other filings with the SEC. Factors that may cause such differences include, but are not limited to, the outcome of any legal proceedings that have been or may be instituted against the company related to the merger between Cytocom and Cleveland BioLabs; unexpected costs, charges or expenses resulting from the merger; our need for additional financing to meet our business objectives; our history of operating losses; our ability to successfully develop, obtain regulatory approval for, and commercialize our products in a timely manner; our plans to research, develop and commercialize our product candidates; our ability to attract collaborators with development, regulatory and commercialization expertise; our plans and expectations with respect to future clinical trials and commercial scale-up activities; our reliance on third-party manufacturers of our product candidates; the size and growth potential of the markets for our product candidates, and our ability to serve those markets; the rate and degree of market acceptance of our product candidates; regulatory requirements and developments in the United States, the European Union and foreign countries; the performance of our third-party suppliers and manufacturers; the success of competing therapies that are or may become available; our ability to attract and retain key scientific or management personnel; our historical reliance on government funding for a significant portion of our operating costs and expenses; government contracting processes and requirements; the exercise of significant influence over our company by our largest individual stockholder; the impact of the novel coronavirus ("COVID-19") pandemic on our business, operations and clinical development; the geopolitical relationship between the United States and the Russian Federation as well as general business, legal, financial and other conditions within the Russian Federation; our ability to obtain and maintain intellectual property protection for our product candidates; our potential vulnerability to cybersecurity breaches; and other factors discussed in our SEC filings, including our Annual Report on Form 10-K for the year ended December 31, 2020 and the risk factors discussed under the heading “Risk Factors” in the proxy statement/prospectus the company filed in connection with the merger on June 10, 2021.
Given these uncertainties, you should not place undue reliance on these forward-looking statements. The forward-looking statements included in this press release are made only as of the date hereof. We do not undertake any obligation to update any such statements or to publicly announce the results of any revisions to any of such statements to reflect future events or developments.
Contacts:
Cytocom, Inc.
Nichol Ochsner
Senior V.P. Investor Relations and Corporate Communications
(732) 754-2545
nichol.ochsner@cytocom.com
Tiberend Strategic Advisors, Inc.
Maureen McEnroe, CFA (Investors)
(212) 375-2664
mmcenroe@tiberend.com
Johanna Bennett (Media)
(212) 375-2686
jbennett@tiberend.com
Cytocom Inc. Provides Updates on Key Clinical Programs for Crohn’s Disease, Hematology, Pancreatic Cancer and COVID-19
Patient enrollment in Phase 3 pediatric Crohn’s disease trial expected to initiate by year-end 2021; End-of-Phase 2 meeting successfully completed in July
Exploring development of entolimod in hematology indications
Plan to initiate a clinical trial exploring CYTO-401 in pancreatic cancer in 1H22
Plan to study CYTO-205 in patients with acute and post-acute COVID-19
FORT COLLINS, Colo., August 9, 2021 /PRNewswire/ -- Cytocom Inc. (Nasdaq: CBLI), a leading biopharmaceutical company creating next-generation immune therapies that focus on immune restoration and homeostasis, today provided an update regarding its portfolio of clinical programs.
“We are excited and believe that we are well positioned to further the development of our clinical-stage pipeline and showcase the power of our expanded post-merger drug development capabilities,” stated Michael K. Handley, President and CEO of Cytocom. “We are strategically focused on immune-modulating treatments designed to address anemia and neutropenia, emergent viruses, cancer, and autoimmune diseases.”
Mr. Handley continued, “The coming months will be busy. We are completing the necessary steps to begin enrolling patients by year-end 2021 in a Phase 3 clinical trial for our lead drug candidate, CYTO-201, for the treatment of Crohn’s disease. Building on our legacy Cytocom pipeline are the Cleveland BioLabs assets, specifically entolimod, an immune-stimulatory agent. We are eager to explore new indications for entolimod and remain excited about the potential for toll-like receptor 5 agonists in treating neutropenia and anemia in cancer patients. Our team is working to put together development plans in hematology and we are in talks with a renowned medical facility to begin a clinical study using entolimod in the coming months.”
Mr. Handley concluded, “In addition, we plan to follow in the first half of 2022 with a clinical trial exploring CYTO-401 as an adjunct to standard of care therapy to extend the duration of disease remission in patients with pancreatic cancer. We are also completing the necessary steps to activate clinical trial sites and begin enrolling patients in clinical trials exploring CYTO-205 in patients with acute and post-acute COVID-19. This could be a particularly compelling opportunity given the pernicious nature of the SARS-CoV-2 virus, which, despite growing vaccination rates, continues to impact millions of people worldwide. What’s more, the medical community has specifically expressed interest on a “long haulers” study.”
CYTO-201 and Crohn's Disease
Cytocom's lead investigational drug candidate, CYTO-201, is being studied for the treatment of pediatric patients with Crohn's disease, an inflammatory bowel disease that causes chronic inflammation of the gastrointestinal (or digestive) tract, causing symptoms such as persistent diarrhea, abdominal pain and rectal bleeding. Studies show that because the signs and symptoms of the disease are unpredictable, patients living with the disease endure significant burdens, not only physical, but also emotional and economic.
On July 27, 2021, Cytocom completed a successful end of Phase 2 meeting with the U.S. Food and Drug Administration (FDA) regarding a clinical development plan for a Phase 3 clinical trial evaluating CYTO-201 in pediatric Crohn’s patients. Cytocom is partnering with ICON plc (NASDAQ: ICLR), a global contract research organization (CRO), to manage the trial, and expects to begin enrolling patients by year-end 2021.
Entolimod and Hematology
Cytocom’s new management team is now reviewing information regarding the past development work for entolimod, as well as previous clinical trial data. The company plans to address the clinical hold imposed by the U.S. Food and Drug Administrations (FDA) in 2019 in order to initiate clinical trials for new indications. In addition to continuing work on the Acute Radiation Syndrome or ARS, Cytocom plans to explore new indications for entolimod based on the potential of toll-like receptor 5 agonists in hematology indications, specifically the treatment of neutropenia and anemia in cancer patients. Cytocom is developing a hematology program and discussions are underway with a renowned academic institution to potentially initiate the first study later this year using entolimod in a hematology indication.
CYTO-401 and Pancreatic Cancer
Cytocom is developing CYTO-401 as an adjunct to standard of care therapy to extend the duration of disease remission in patients with pancreatic cancer. In August 2021, the company received FDA feedback regarding the clinical development program. Having feedback on the development program and establishing an advisory panel of oncology experts, Cytocom is now evaluating contract research organizations (CROs) and is planning to initiate the clinical trial in the first half of 2022.
CYTO-205 and Acute and Post-Acute COVID-19
With the FDA having cleared an Investigational New Drug (IND) application, Cytocom expects to begin a Phase 2 clinical trial in the third quarter of 2021 to evaluate the safety and efficacy of CYTO-205 to slow or halt the progression of the SARS-CoV-2, the virus that causes COVID-19.
Cytocom is finalizing protocols for a study evaluating CYTO-205 in patients with post-acute COVID-19 syndrome (PACS). Also known as “long haulers,” these patients represent a high unmet medical need with roughly 30% of all COVID-19 infections developing into long-haul syndrome. Cytocom plans to conduct the trial under the existing IND and expects to begin enrolling patients by year-end 2021. CYTO-205 is designed to modulate immune system function by decreasing elevated inflammatory responses associated with viral infection.
About Cytocom
Cytocom Inc. is a clinical-stage biopharmaceutical company developing novel immunotherapies targeting autoimmune, neutropenia/anemia, emerging viruses and cancers based on a proprietary platform designed to rebalance the body’s immune system and restore homeostasis. The company also has one of the largest platforms of toll-like immune receptors (TLR4, TLR5 and TLR9) in the biopharmaceutical industry, addressing conditions such as radiation sickness and cancer treatment side effects. Cytocom is developing therapies designed to elicit directly within patients a robust and durable response of antigen-specific killer T-cells and antibodies, thereby activating essential immune defenses against autoimmune, inflammatory, infectious diseases, and cancers. Specifically, Cytocom has several clinical-stage development programs for Crohn’s disease, hematology, pancreatic cancer, and COVID-19 in addition to expansion to fibromyalgia and multiple sclerosis. To learn more about Cytocom Inc., please visit www.cytocom.com.
Forward Looking Statements:
This press release contains forward-looking statements that involve risks and uncertainties. All statements other than statements of current or historical fact contained in this press release, including statements regarding the expected clinical development timeline for its product candidates. The words "anticipate," "believe," "continue," "should," "estimate," "expect," "intend," "may," "plan," "project," "will," and similar expressions, as they relate to us, are intended to identify forward-looking statements. We have based these forward-looking statements on the current expectations about future events held by management. While we believe these expectations are reasonable, such forward-looking statements are inherently subject to risks and uncertainties, many of which are beyond our control. The company’s actual future results may differ materially from those discussed here for various reasons. The company discusses many of these risks under the heading "Risk Factors" in the proxy statement/prospectus filed with the SEC on June 10, 2021, as updated by the company’s other filings with the SEC. Factors that may cause such differences include, but are not limited to, the outcome of any legal proceedings that have been or may be instituted against the company related to the merger with Cleveland BioLabs; unexpected costs, charges or expenses resulting from the merger; our need for additional financing to meet our business objectives; our history of operating losses; our ability to successfully develop, obtain regulatory approval for, and commercialize our products in a timely manner; our plans to research, develop and commercialize our product candidates; our ability to attract collaborators with development, regulatory and commercialization expertise; our plans and expectations with respect to future clinical trials and commercial scale-up activities; our reliance on third-party manufacturers of our product candidates; the size and growth potential of the markets for our product candidates, and our ability to serve those markets; the rate and degree of market acceptance of our product candidates; regulatory requirements and developments in the United States, the European Union and foreign countries; the performance of our third-party suppliers and manufacturers; the success of competing therapies that are or may become available; our ability to attract and retain key scientific or management personnel; our historical reliance on government funding for a significant portion of our operating costs and expenses; government contracting processes and requirements; the exercise of significant influence over our company by our largest individual stockholder; the impact of the novel coronavirus ("COVID-19") pandemic on our business, operations and clinical development; the geopolitical relationship between the United States and the Russian Federation as well as general business, legal, financial and other conditions within the Russian Federation; our ability to obtain and maintain intellectual property protection for our product candidates; our potential vulnerability to cybersecurity breaches; and other factors discussed in our SEC filings, including our Annual Report on Form 10-K for the year ended December 31, 2020 and the risk factors discussed under the heading “Risk Factors” in the proxy statement/prospectus the company filed in connection with the merger on June 10, 2021.
Given these uncertainties, you should not place undue reliance on these forward-looking statements. The forward-looking statements included in this press release are made only as of the date hereof. We do not undertake any obligation to update any such statements or to publicly announce the results of any revisions to any of such statements to reflect future events or developments.
Contacts:
Cytocom, Inc.
Nichol Ochsner
Senior V.P. Investor Relations and Corporate Communications
(732) 754-2545
nichol.ochsner@cytocom.com
Tiberend Strategic Advisors, Inc.
Maureen McEnroe, CFA (Investors)
(212) 375-2664
mmcenroe@tiberend.com
Johanna Bennett (Media)
(212) 375-2686
jbennett@tiberend.com
And down goes imun, and down goes cbli/cyto.
because CYTO is already used. Altamira Therapeutics
I wonder why it will still trade under CBLI not CYTO
Cytocom Inc. Announces Completed Merger with Cleveland BioLabs
New company to operate as “Cytocom, Inc.” with its common stock traded on Nasdaq
FORT COLLINS, Colo., July 28, 2021 /PRNewswire/ -- Cytocom Inc. (“Cytocom” or “Company”), a leading biopharmaceutical company creating next-generation therapies that focus on immune homeostasis, today announced the completion of its merger with Cleveland BioLabs, Inc. The all-stock transaction was first announced on October 19, 2020.
Shares of the new Cytocom’s common stock will begin trading on Nasdaq under the ticker symbol “CBLI,” at the opening bell on Wednesday, July 28, 2021.
As previously reported, the newly combined company will operate as “Cytocom, Inc.” under the leadership of the existing Cytocom management team led by CEO Michael K. Handley. Mr. Handley now serves as the new company’s President and CEO.
“With the merger completed, we look forward to further advancing our late-stage clinical programs and expanding what we believe to be one of the largest toll-like receptor platforms in the industry,” said Michael K. Handley. “This merger, coupled with our acquisition of ImQuest Life Sciences and the listing of the new Cytocom common shares on Nasdaq, represents a transformative growth opportunity and fits firmly with our goal of becoming a recognized leader in immune-modulating therapies targeting cancer, ARS, inflammatory and autoimmune diseases, and viruses, including COVID-19. Looking forward, we anticipate achieving multiple commercial, regulatory and clinical milestones over the next 12 to 18 months that should enable us to showcase the power of our drug development platform and further generate shareholder value.”
McGuireWoods LLP represented Cleveland BioLabs and Troutman Pepper Hamilton Sanders LLP represented Cytocom in the merger.
About Cytocom
Cytocom, Inc. is a clinical-stage biopharmaceutical company developing novel immunotherapies targeting autoimmune, inflammatory, infectious diseases and cancers based on a proprietary platform designed to rebalance the body’s immune system and restore homeostasis. The company also has one of the largest platforms of toll-like immune receptors (TLR4, TLR5 and TLR9) in the biopharmaceutical industry, addressing conditions such as radiation sickness and cancer treatment side effects. Cytocom is developing therapies designed to elicit directly within patients a robust and durable response of antigen-specific killer T-cells and antibodies, thereby activating essential immune defenses against autoimmune, inflammatory, infectious diseases, and cancers. Specifically, Cytocom has several clinical-stage development programs for Crohn’s disease, fibromyalgia, multiple sclerosis and pancreatic cancer. To learn more about Cytocom, Inc., please visit www.cytocom.com.
Forward Looking Statements:
This press release contains forward-looking statements that involve risks and uncertainties. All statements other than statements of current or historical fact contained in this press release, including statements regarding the future financial position, business strategy, new products, budgets, liquidity, cash flows, projected costs, regulatory approvals, the impact of any laws or regulations applicable to the company, and plans and objectives of management for future operations, are forward-looking statements. The words "anticipate," "believe," "continue," "should," "estimate," "expect," "intend," "may," "plan," "project," "will," and similar expressions, as they relate to us, are intended to identify forward-looking statements. We have based these forward-looking statements on the current expectations about future events held by management of both companies. While we believe these expectations are reasonable, such forward-looking statements are inherently subject to risks and uncertainties, many of which are beyond the control of either company. The company’s actual future results may differ materially from those discussed here for various reasons. The company discusses many of these risks under the heading "Risk Factors" in the proxy statement/prospectus filed with the SEC, as updated by the company’s other filings with the SEC. Factors that may cause such differences include, but are not limited to, the outcome of any legal proceedings that have been or may be instituted against the company related to the merger agreement or the Merger; unexpected costs, charges or expenses resulting from the Merger; our need for additional financing to meet our business objectives; our history of operating losses; our ability to successfully develop, obtain regulatory approval for, and commercialize our products in a timely manner; our plans to research, develop and commercialize our product candidates; our ability to attract collaborators with development, regulatory and commercialization expertise; our plans and expectations with respect to future clinical trials and commercial scale-up activities; our reliance on third-party manufacturers of our product candidates; the size and growth potential of the markets for our product candidates, and our ability to serve those markets; the rate and degree of market acceptance of our product candidates; regulatory requirements and developments in the United States, the European Union and foreign countries; the performance of our third-party suppliers and manufacturers; the success of competing therapies that are or may become available; our ability to attract and retain key scientific or management personnel; our reliance on government funding for a significant portion of our operating costs and expenses; government contracting processes and requirements; the exercise of significant influence over our company by our largest individual stockholder; the impact of the novel coronavirus ("COVID-19") pandemic on our business, operations and clinical development; the geopolitical relationship between the United States and the Russian Federation as well as general business, legal, financial and other conditions within the Russian Federation; our ability to obtain and maintain intellectual property protection for our product candidates; our potential vulnerability to cybersecurity breaches; and other factors discussed in our SEC filings, including our Annual Report on Form 10-K for the year ended December 31, 2020and the risk factors discussed under the heading “Risk Factors” in the proxy statement/prospectus the company filed in connection with the merger.
Given these uncertainties, you should not place undue reliance on these forward-looking statements. The forward-looking statements included in this press release are made only as of the date hereof. We do not undertake any obligation to update any such statements or to publicly announce the results of any revisions to any of such statements to reflect future events or developments.
$3.50 now, look out below if this goes below $1. And to think there were actually predictions of $160 to $240 for imun. Of course no one took them seriously. Sorry for anyone who bought into the merger hype and chased imun at above $20 (.02 before the ridiculous 1:1000 reverse split). Obviously the math did not add up.
Were you looking for the move down towards $3.50 as I predicted? Keep watching.
Right now it shows 204 shares traded at $3.90 - hard to believe anyone was ever predicting $160 for this little scammer.
Is etrade showing correctly? Shows 0.00 open and no trading today.
Seriously? Quoting an old imun p/r that touted a newly hired board member even though that board member along with the entire board and ceo have driven this train wreck of a 1:1000 reverse split and loss of more than 80% from recent highs? And all the while not a word to shareholders about it.
There is no defense for what is going on here. Some people must simply like to lose money.
I want to see some development as well, but I give them still a credit. I do think, that Mr. Phelps is not rising his very good reputation playing games. He needs more time to get things going. I still hope so..
Kevin J Phelps
https://www.globenewswire.com/en/news-release/2019/01/09/1683002/0/en/Immune-Therapeutics-welcomes-Kevin-Phelps-to-Board-of-Directors.html
Kevin Phelps is a finance and business development professional who has spent his career determining, satisfying and managing the operational needs of a wide range of companies. With greater than 25 years of broad, professional corporate experience, Mr. Phelps has held senior management positions, successfully raising capital for startup companies, managing financial organizations and developing new businesses through technology, strategic alliances and acquisition projects.
Mr. Phelps is a General Partner in Trillium Group, LLC, a Rochester, New York based venture capital firm and a Founder of Cashel Rock Advisors and FinanciaLink Strategic Alliances, two private wealth management firms specializing in strategies for corporations and high net-worth individuals.
His professional experience began as a CPA with Price Waterhouse (now PwC), where he consulted with over 20 companies with a principal focus on emerging growth opportunities. In 1987, he was recruited to head financial planning for Eastman Kodak’s BioProducts Division. He assisted in the spinoff of the business into an international joint venture and became the Chief Financial Officer of the new entity, Genencor International, Inc. In this role, he created and managed Genencor’s finance and treasury groups and established the company’s accounting and reporting practices. He raised in excess of $100 million in debt capital to fund Genencor’s operations and expansion. He also served as Vice President of New Business Development.
“We are very pleased to welcome Mr. Phelps to our Board of Directors. His extensive experience as a finance specialist, along with his deep knowledge of business development and operations, will assist the company in moving to the forefront of specialized pharmaceuticals,” stated Noreen Griffin, President and CEO of Immune Therapeutics, Inc.
“I am excited to join Immune Therapeutics’ Board of Directors and to contribute my experience in entrepreneurial ventures. Immune Therapeutics has great potential, and I intend to assist in maximizing this potential to bring unprecedented ROI for investors,” said Mr. Phelps.
“I look forward to working with Mr. Phelps. Mr. Phelps’s proven leadership in business and finance make him an excellent addition to IMUN’s Board,” stated Dr. Roscoe Moore, Chairman of Immune Therapeutics’ Board of Directors, member of the company’s Scientific Advisory Board, and Former United States Assistant Surgeon General.
Pretty obvious that management has no intention of growing this company and all they will want to do is sell off their shares little by little from $6 on down using whatever little hype can come from the Cytocom share ownership until this is right back to 2 cents.
Remember I believe their shares were split protected and also remember Handley left this company to go to Cytocom.
Complete and utter scam. And we all know who is behind it.
Noreeeeeeeeeeeeeeen.
Absolutely correct! Something has been seriously wrong here, actually very, very seriously wrong for a long, long time!
Below $3 even faster than I would have thought. I realize it can be difficult to face the truth, but it's time to stop with all the excuses and start getting to the bottom of this scam. What does the ceo have to say? Something seriously wrong here.
Down from $30 and ridiculous predictions of $160 to $240 - sounds like quite the downdraft. An "uplift" of 100% wouldn't even get close to gaining back recent huge losses.
Trading in otc pink is nothing wrong with it. The company eliminated all the toxic financing and has raised enough money to uplift.
IMUN management is a complete embarrassment and they completely fleeced believers in the company.
I will give them credit for the lack of dilution so far, but everyone knows that is coming sooner or later. And as I said months ago, and is THE MOST crucial point here, OTC investors are not going to buy a stock priced over 25 cents, let alone $5 like what IMUN is near. Just will not happen. Hence the complete lack of volume here. Most OTC traders barely even buy a stock over a penny for that matter.
So the decision to RS this was just complete idiocy. They are on the OTC-a PENNY STOCK market. Not a DOLLAR market. They did not play to their demographic...penny stock traders. If they would have RS to dollars and somehow someway been able to uplist right away? Ok. But you don't RS and then stay on the OTC penny market where NO ONE buys stocks over a dollar.
Pure stupidity.
Made no sense then and still doesn't. This will likely hover where it is now, maybe have a small spike now and then, but dilution will eventually take over and this will be back to .03 within a year which will be a 99.999999999999% loss from here.
And yet cbli down below $5 from $17 and imun below $5 from $30 and the asinine predictions of $160 to $240.
The company could acquire cyto after the merger gets completed.
Sparta Healthcare Acquisition Corp. (SPTA)
Sparta Healthcare Acquisition will go public soon, but the exact IPO date is still unknown.
Stock Price: $10.00
https://stockanalysis.com/stocks/spta/company/
https://sec.report/Document/0001140361-21-018842/
I find that very interesting., maybe they have some intention to get IMUN acquired
https://www.nasdaq.com/market-activity/ipos/overview?dealId=1160977-97730
interesting to read the company description...
SPTAU CYTO both of these companies have the same CEO Michael Handley and strong backing of investment banking Kingswood Capital & Bridgeway Capital Partners
Very bullish for CBLI snd IMUN shareholders!
Biotech-focused SPAC Sparta Healthcare Acquisition files for a $100 million IPO
Sparta Healthcare Acquisition is led by CEO and Chairman Michael Handley, who has served as the CEO of Cytocom since 2020 and has assisted in or led the global commercialization of 17 devices or drugs over the course of his career, and CFO Nicholas Hemmerly, the current Head of Investment Banking at Bridgeway Capital Partners. The company plans to leverage management's experience and target the North American biotech and therapeutic sectors, focusing on businesses with mid-to-late stage therapeutic candidates across a variety of indications.
|
Followers
|
136
|
Posters
|
|
|
Posts (Today)
|
0
|
Posts (Total)
|
18220
|
|
Created
|
03/09/06
|
Type
|
Free
|
| Moderators | |||
IMUN - Immune Therapeutics
| Shareholders of Record | 808 | a/o Apr 16, 2019 |
|
| Authorized Shares | 750,000,000 | a/o May 4, 2020 | |
| Outstanding Shares | 457,577,799 | a/o May 4, 2020 |
Quick Links:
www.immunetherapeutics.com
www.cytocom.com (subsidiary of IMUN - Cytocom will get eventual U.S. sales - if that ever happens - hopefully in 2-3 years?? IMUN owns 16.95% of Cytocom as of June 30, 2018. LDN has already received various Phase 2 approvals in the United States, see www.cytocom.com/investors/)
www.fidson.com (partner in Nigeria for marketing and distribution)
http://www.acromaxdominicana.com/ (partner for manufacturing in Dominican Republic - contract in 8-k)
http://www.omaera.com/ (partner in Kenya for marketing and distribution)
www.aharpharma.com (partner in Nigeria)
Important Links:
HIV Eradication 7 minute video, Oyagen
Kevin Phelps, CFO of Oyagen
Kevin Phelps (already on board of IMUN) becomes Interim CEO of IMUN, Michael Handley focusing on role as CEO of Cytocom and Forte Biotechechnology
https://m.youtube.com/watch?v=iydWaAmHblM
Youtube Video on Covid19 and low dose naltrexone
IMUN and Cytocom will collaborate to develop therapies for COVID19
IMUN provides guidance on reverse stock split and name change (reverse stock split first announced in 2018)
ImQuest Biosciences
Patent
IMUN appoints Michael Sander to board
Michael Handley is new CEO and president of IMUN
IMUN adds Kevin Phelps to the Board of Directors
Research on Lodonal in A & U Magazine
10-q from Q3 2018: IMUN ships first 250k pills to Fidson Healthcare (pg 25)
Ambassador Jack Brewer joins IMUN's board of directors
IMUN labeled as Pioneer in article in Forbes magazine
Dr Gary Blick, renown HIV specialist, joins IMUN's scientific advisory board
IMUN adds former US Assistant Surgeon General, Dr Roscoe Moore, as the new Chairman of the Board
Video from AIDS 2018 conference - starts at 59:58
IMUN acquires 10% stake in Cytocom
Research on Lodonal for HIV featured in Plus Magazine
Link to HIV Plus Magazine article on Lodonal
IMUN announces publication of White Paper on Lodonal
8-k from 5/22/18: Fidson Healthcare accepts its appointment as the sole distributor of Lodonal in Nigeria
CNN Video interviewing Fidelis Ayebae, CEO of Fidson Healthcare
Form SC 13G from 5/15/18: Iliad Research and Trading owns 9.99% stake in Immune Therapeutics
8-k from 4/27/18: Kenya Pharmacy and Poison board files NDA for Lodonal for final review
IMUN received minutes from FDA Meeting for Lodonal for Crohn's Disease
First order shipped. First revenues for IMUN for $195k from AHAR Pharma
System for Award Management (companies need to register here to qualify for UNAIDS and other goverment grants) - Click on search records, then type in Immune Therapeutics.
8-k from 12/1/17: Shareholder letter - AHAR issues IMUN its first ever purchase order of $195,000
10-Q from 11/14/17: IMUN lists $214k in inventory. First time ever listing having inventory on a 10-q or 10-k in IMUN's history
8-k from 11/2/17 - Note with Iliad Research and Trading
8-k from 9/8/17: IMUN announces exclusive agreement with Omaera Pharmaceuticals for Lodonal in Kenya - valued at a minimum of $31m in revenue over 3 years - first purchase order contractually guaranteed by 11/22/17
Kenya Agriculture, Fish, and Food Authorities: Approved List of Suppliers, Human Drugs and Medicine - Omaera Pharmaceuticals is on the list
8-k from 8/25/17: IMUN hires new COO Rudy Williams and hires new CEO of Cytocom Dr. John H Abeles (Dr. John H Abeles will also be new senior executive advisor of IMUN)
10-Q from 8/14/17: IMUN records first GAIN ever in shareholder equity for a quarter, and achieves this on NO REVENUE (due to elimination of toxic debt)
8-k from 4/27/16: LDN is approved in Nigeria
"The NAFDAC approval follows the successful completion of a 90-day bridging study conducted by AHAR Pharma on behalf of the Company in conjunction with State Specialist Hospital in Asubiaro. That study, “ A Bridging Study to Evaluate the Effects of ‘Lodonal’ as an Immune-System regulating Agent in Subjects in which Their Immune System is Compromised: Lodonal in the Treatment of Subjects with Human Immuno-Deficiency virus (HIV), ” met the primary and secondary endpoints for both efficacy and safety.
Breakthrough Lodonal Results on HIV Patients:
The 90-Day Bridging Study was undertaken at the State Specialist Hospital in Asubiaro, Osogbo, Osun State, Nigeria and the primary objective of this Bridging trial was to confirm that Lodonal had a beneficial effect on the immune system of immune deficient patients and safety. This was a single center, open labeled, randomized, bridging study of a 150 people. The Treatment Group was treated with 4.5 mg of Lodonal nightly in conjunction with antiretroviral. The Control Group was treated with antiretroviral plus placebo . The primary endpoints were efficacy and safety determined by a minimum increase of 25% in the CD4 count with no adverse effects on quality of life.
The results yielded an average 44% increase in CD4 count in the Treatment Group compared with 11% increase for the Control Group and there was no adverse effect on quality of life or opportunistic infections during the trial. The Nigeria trial’s results were consistent with previous clinical trials of LDN.
Due to its favorable cost/benefit, the Company believes Lodonal has large market potential to be the first affordable non-toxic therapy of its kind. It is administered in a single oral dose daily making medical and drug compliance much easier and is designed to produce a significant reduction in opportunistic infections. In addition to affordably improving the quality of patients’ lives, it offers a compelling economic benefit to health care systems."
8-k from 5/23/17: LDN gets marketing approval in Nigeria
CNN interviews Fidson Healthcare CEO, Fidelis Ayebae
8-k from 11/2/16: Contract with IMUN's manufacturer in the Domincan Republic, Acromax Dominicana
8-k from 4/13/17: Debt restructuring to eliminate a very "toxic" note.
8-k from 3/14/17 - Debt restructuring with Florida Hedge fund
8-k/a from 3/17/17 - Debt restructuring with Florida Hedge fund - ammendment
8-k from 11/15/16 - Debt restructuring
8-k from 4/28/17: IMUN files NDA for LDN for HIV and Cancer in Kenya
PR from 5/31/17: IMUN signs MoU with Ivory Coast for LDN
PR from 10/26/16: IMUN files NDA for LDN for HIV, Cancer and as an Immune Booster with Senegal
PR from August 2015: Letter of Intent from Fidson Healthcare PLC - this publishing popped IMUN to just over $0.30 briefly
Stock Quote of Fidson Healthcare PLC - IMUN's supposed partner (LOI) for marketing / distribution in Nigeria - notice how the ticker has shot up since April
8-k from 4/13/16: IMUN Provides Drug Development Status on MENK (Methionine-Enkelphine) in China
8-k from 4/21/16: IMUN signs binding LOI to acquire Chinese CAR-T Technology and Clinical data
Nigeria Clinical Trials Registry - Lodonal - note that it still says "Pending". According to 8-k's, the drug is officially approved, so IMUN should be good on this
Low Dose Naltrexone Forum - Proboards
LDN Clinical Trials via ldninfo.org (has not been updated since 2013, but still a very nice list)
List of Clinical Trial Publications for LDN via clinicaltrials.gov
www.lowdosenaltrexone.org
11 Facts about HIV in Africa
http://www.ldnafricaaids.org/ (has not been updated since late 2012)
http://www.ldnafricaaids.org/wp-content/uploads/2011/08/Traore-et-al-1.pdf (clinical study on LDN in Mali)
https://www.usaid.gov/work-usaid/get-grant-or-contract/grant-and-contract-process
http://data.unaids.org/publications/irc-pub05/jc431-stratplan4_en.pdf
http://www.ldnafricaaids.org/?page_id=3 (the story of LDN and the late Dr. Bernard Bihari (passed away May 2010), the pioneer of low dose naltrexone - TNIB / IMUN acquired patents in late 2012/early 2013 - see 10-k)
The medicine your doctor never told you about (but should have)
Low-Dose Naltrexone (LDN): One of the RARE Drugs that Actually Helps Your Body to Heal Itself
My experience with Low Dose Naltrexone By David Gluck, MD (the story of a lifelong friend of Dr. Bernard Bihari, the pioneer of LDN)
We now have a NEW Senior Regulatory Advisor (see last page of powerpoint) who is (or was?) ALSO the Senior Regulatory Officer for the GATES FOUNDATION! - other than the powerpoint presentation from the LD Micro conference, there has not yet been any 8-k recognizing any of these new additions (Dr. John H Abeles, Rufus (Rudy) Williams, Vincent Ahonkhai).
Speeding Access to Low and Middle Income Countries: by Vincent Ahonkhai
PEPFAR - Department of Defense
http://naca.gov.ng/
http://www.nascop.or.ke/
http://pharmacyboardkenya.org/
http://www.croiconference.org/search/node/naltrexone
https://www.ldnscience.org/research
Videos to watch on Low Dose Naltrexone
https://www.youtube.com/watch?v=Kz52KK5IhOc&t=1s (4 mins, CBS News - Wonder drug Low Dose Naltrexone?...very uplifting encouraging video about a woman's story on LDN)
In this 4 min video above, it states that no Big Pharma companies are backing LDN. This is correct, because it is off-label: 50-mg Naltrexone was approved by the FDA in the 80's. Thus, making it cheap because 50-mg Naltrexone is generic (50mg Naltrexone didn't work well for what it was approved for - heroin addicts, therefore it ended up becoming generic). Low dose naltrexone, 4.5mg, however, is patented, and studies have shown its effectiveness (44% increase in CD4 count in Nigerian trials, when they were searching for a minimum of 25% increase in CD4 count). And it's safe - very little side effects except for possible lucid dreams. But still, it's "cheap". Noreen says it will costs patients in Nigeria $.84 per day, $0.24 of which will be profit. The low cost is the reason why big pharma did not WANT to fund this drug. Because there has always been little research done on LDN, big pharma was happy keeping it that way. They knew that if LDN did ever gain traction as the go-to therapy for any given disease, it would make big pharma lose billions of dollars, if in fact LDN can replace more expensive drugs that the big pharma companies are selling. This could potentially make IMUN a potential acquisition target by big pharma in the future.
https://www.youtube.com/watch?v=FDCn0JWv6Io (59 mins; very informative documentary telling the story of LDN)
https://www.youtube.com/watch?v=pg1T0bKxx9E [11 mins; a woman gives a detailed explanation of how LDN has helped her - 4 month update (not doing so well) and 1 year update (doing much better)]
https://www.youtube.com/watch?v=rll1A3aFhjc (79 mins; "[the late] Dr Bihari [the pioneer of low dose naltrexone] is Interviewed about Low Dose Naltrexone LDN for Normalizing Immune System Function")
https://www.youtube.com/watch?v=r4quvjDiXcY (60 mins; LDN & Cancer - The Game Changer)
https://www.youtube.com/watch?v=l8sWzoLtop4 (29 mins; LDN as a treatment for Autoimmune disease)
https://www.youtube.com/watch?v=Aaqa50lcoeA&t=133s (Dr. Jill Smith from Penn State talks about Low Dose Naltrexone for Crohn's disease)
Books on Low Dose Naltrexone (great reviews on Amazon)
https://www.amazon.com/Promise-Low-Dose-Naltrexone-Therapy/dp/0786437154/ref=sr_1_2?ie=UTF8&qid=1497648079&sr=8-2&keywords=low+dose+naltrexone
https://www.amazon.com/LDN-Book-Little-Known-Naltrexone-Revolutionize/dp/1603586644/ref=pd_bxgy_14_img_2?_encoding=UTF8&pd_rd_i=1603586644&pd_rd_r=GPZPCV3F5HAS56V1CT2F&pd_rd_w=NLw9g&pd_rd_wg=ExBQX&psc=1&refRID=GPZPCV3F5HAS56V1CT2F
https://www.amazon.com/Honest-Medicine-Time-Tested-Inexpensive-Life-Threatening/dp/0982969015/ref=sr_1_1?ie=UTF8&qid=1500958081&sr=8-1&keywords=honest+medicine
Upcoming Catalysts (expected)
- Contracts with Fidson Healthcare and other distributors in Nigeria and potentially other African nations.
- Approval from NACA, NASCOP.
- Revenues beginning with Fidson Healthcare (Nigeria) and other distributors.
- Revenues beginning with Omaera Pharmaceuticals (Kenya).
- Completion of Lodonal White paper.
- Lodonal expected to be included on UNAIDS essential medicines list.
- Approval in Ivory Coast.
- Grant approvals from UNAIDS and / or other goverment organizations such as USAID / Gates / PEPFAR (not exactly clear what is going on with this), but IMUN is indeed registered with sam.gov (System for Award Management), which is where a company must register to get goverment grants. They registered in January 2018.
Clinical Trials: Crohn’s Disease
The first clinical trial results with LDN for immune disorders were published only recently in a peer-reviewed medical journal in 2007 which evaluated LDN treatment in a pilot Phase II study of 17 patients with moderate to severe, active Crohn’s disease. The open-label pilot study was conducted by Penn State University to evaluate LDN response, safety and toxicity. Patients were treated with LDN orally each evening at a dose of 4.5 mg for 3 months. A total of 17 patients were enrolled, 16 of whom completed the study. No laboratory abnormalities were noted. The most common side effect was sleep disturbances (occurred when dosing at night, at about bed-time), occurring in seven patients (41%). Two-thirds of patients in this study went into remission after 4.5 mg daily LDN treatment (p < 0.001), with 89% of patients overall showing some degree of response. Blood inflammatory markers were also analyzed, specifically c-reactive protein, or c-RP and erythrocyte sedimentation rate, or ESR. C-RP levels decreased from a median value of 2.6 (normal <0.8) at baseline to a value of 0.9 by the twelfth week of treatment, which was statistically significant (p = 0.03). The ESR decreased from a mean baseline value of 23.3 ± 0.4 mm/h to 17.9 ± 0.3 mm/h, which was also significant (p = 0.04). Baseline plasma enkephalin levels were 9.5 ± 2.8 pg/mL, and decreased to a value of 3.6±1.0 pg/mL at week 12 of LDN therapy.
A second clinical study was conducted by Penn State University as a randomized double-blind, placebo-controlled study to test the efficacy and safety of LDN for 12 weeks in adults with moderate to severe active Crohn’s disease. Forty subjects were enrolled in the study. Randomized patients received daily oral administration of LDN (4.5 mg/day) or placebo. Fatigue was the only side effect reported of statistical significance, and it was greater in subjects receiving placebo. Thirty percent of patients in this study went into remission (defined as a Crohn’s Disease Activity Index (“CDAI”) score ≤ 150), with 88% of patients overall showing some degree of response. Blood inflammatory markers, c-RP and ESR, were analyzed. Patients who exhibited a 70-point drop in CDAI scores with LDN had higher c-RP values at baseline (2.0 ± 0.5 mg/dl) compared to those subjects on LDN who did not exhibit a response (0.8 ± 0.3 mg/dl); however, this difference was not statistically significant (all values were included in the analysis including those subjects who flared during the study). Based upon this data, patients with high c-RP levels may be more likely to demonstrate a clinical response to treatment with LDN.
| 13 | P a g e |
A pilot Phase II clinical trial was conducted by Penn State University in children with moderate to severe active Crohn’s disease. Fourteen subjects were enrolled, 12 subjects were randomized and treated with a mean age of 12.3 years (range 8-17 years). Children were randomized to placebo or LDN (0.1 mg/kg or a maximum dose of 4.5 mg) orally for 8 weeks followed by open-label treatment for an additional 8 weeks of LDN at the same dose of 0.1 mg/kg or 4.5 mg. Results showed a significantly greater reduction of baseline pain in those taking LDN than in those taking placebo (28.8% reduction versus 18.0% reduction; p = 0.016). LDN was also associated with improved general satisfaction with life (p = 0.045) and with improved mood (p = 0.039), but not improved fatigue or sleep. Thirty-two percent of participants met the criteria for response (defined as a significant reduction in pain plus a significant reduction in either fatigue or sleep problems) during LDN therapy, as contrasted with an 11% response rate during placebo therapy p = 0.05). LDN was rated equally tolerable as placebo and no serious side effects were reported. Laboratory parameters for inflammation, specifically white blood count, c-RP and ESR all improved from baseline to Week eight; however, the results were not significant.
An open-label Phase II trial was conducted for Naltrexone as Therapy for Inflammatory Bowel Disease: Ulcerative Colitis and Crohn’s Disease Twelve patients received naltrexone 4.5 mg/day. Duration (mean ±SD) of naltrexone treatment was 46 ±75 weeks (maximum 270 weeks). One patient withdrew after 8 weeks owing to insomnia. Positive clinical responses were reported in 6/12 patients. Two clinical responders had colonoscopy before and after naltrexone and each had complete mucosal healing. (Leonard B. Weinstock, MD, FACG Journal of Clinical Gastroenterology: September 2014 - Volume 48 - Issue 8 - p 742)
Low Dose Naltrexone Reduces In Vitro Endoplasmic Reticulum Stress and Stimulates Wound Healing in Intestinal Epithelial Cells: A total, 40 patients (43% male, median age 40y, IQR 28–52 years) were treated with 5 mg NTX/day. Response was seen in 23 patients (13 CD; 10 UC), with a median duration of 2 months Conclusion
Low Dose Naltrexone may have a beneficial effect in the treatment of IBD by directly stimulating epithelial wound healing and reducing intestinal ER stress. G. Fuhler*, M. Lie, P. Dimitrijevic, C. J. van der Woude, M. Peppelenbosch Erasmus MC University Medical Centre Rotterdam, Gastroenterology and Hepatology, Rotterdam, Netherlands
Low Dose Naltrexone in the treatment of Crohn’s Disease open label 17 patients were treated with LDN for 12 weeks and showed a 70% reduction in CDAI score with endoscopy 88% in the LDN group and 28% in the control group endoscopic remission 33% in the LDN group 8% in the control group with no adverse events. 10 Dig Dis Sci 2011 Jul;56(7):2088-9 7Michael Arata, MD.
Low Dose Naltrexone in the Treatment of Crohn’s Disease: A Case Series 56 patients on 4.5mg IBS patients show clinical response to LDN (54%) and should be considered as an adjunct to conventional therapy in patients with resistant disease or as a bridge while another treatment plan is formulated. Further studies are required to evaluate the degree of mucosal healing while on LDN. University of Florida http://www.gastrojournal.org/article/S0016-5085(15)32952-8/abstract.
Clinical Trials: HIV/AIDS
A single blind 90 day with 4 week f/u randomized completed a 90-day bridging trial for the treatment of patients with HIV AIDS. The National Agency for Food and Drug Administration and Control (NAFDAC) approval is based on previous clinical data and the Nigeria Bridging Trial. The trial was a single center, open labeled, randomized, bridging study consisting of one hundred and fifty [150] patients of both genders between the ages of 18-60, each of whom was infected with the human immunodeficiency virus (HIV).
The 90-Day Bridging Trial was undertaken at the State Specialist Hospital in Asubiaro, Osogbo, Osun State, Nigeria with the primary objective to confirm Lodonal™ has a beneficial effect on the immune system of immune deficient patients and that it is safe. The trial separated the patients into a Control (placebo) Group and a Treatment Group (which was administered Lodonal™). The efficacy of increasing CD4 count [cell/mm3] between Day-1 and Day-90 by at least 25% was set as the criteria for demonstrating beneficial effect on the immune system. Safety was demonstrated through quality of life assessment and vitals both of which were not adversely affected. Treatment Group patients were given a daily dose of 4.5-mg/kg of Lodonal™.
| 14 | P a g e |
The results yielded an average increase of 44% increase in CD4 count in the Lodonal™ Treatment Group compared to an 11% increase in the Control Group. Additionally, there were no reported opportunistic infections and no toxicity levels uncovered. Liver function remained normal and there was no negative impact on other systems based on blood results. No significant sleep disturbance or vivid dreams were present enough to justify trial discontinuation. No significant adverse CNS, renal, cardiac, hepatic, musculoskeletal, hematopoietic side effects were present. NAFDAC issued drug and marketing approval of Lodonal™ in July of 2017 in the management of HIV/AID’s and immune dysfunction.
A single blind nine-month randomize clinical trial and a single prospective cohort study were conducted in Mali to evaluate the impact of LDN on asymptomatic HIV+ adults. Results of the nine-month study showed an improvement in cluster of differentiation 4 (“CD4”) count in the treatment groups that was significantly greater than the control group at 6 months (p = 0.041) and marginally at 9 months (p = 0.067). Results of the single prospective cohort study showed 71% of subjects that completed the study did not show any indication of clinical AIDS symptoms, side effects or a loss of CD4 count that would warrant initiation of antiretroviral therapy (“ART”) medication.
A 12-week, placebo-controlled trial of LDN was conducted from 1985-1986 in 38 patients with AIDS by Dr. Bihari and his colleagues. Patients who participated in this trial showed a significant difference in the incidence of opportunistic infections with 5 out of 16 patients (31%) on placebo developing opportunistic infections in comparison to 0 of the 22 patients in the LDN group. Other differences between placebo and LDN treated patients included: lymphocyte mitogen responses declined on placebo and not on LDN; pathologically elevated levels of acid-labile alpha interferon declined significantly in the patients on LDN and not in those patients on placebo.
After the conclusion of the above clinical trial, Dr. Bihari began to use LDN in his own medical practice. Of 158 patients in his practice that were evaluated, only ten (6%) were on antivirals. Patients of Dr. Bihari who had taken the drug regularly as prescribed showed no drop in CD4 cells. The average CD4 number in these patients before starting LDN was 358, and the average 18 months later increased to 368. There were 55 patients who had not taken the drug, or had taken it only sporadically (non-compliant). These patients showed a drop of CD4 cells from an average of 297 to 176 in 18 months. This represented a drop in CD4 of approximately 80 per year, which corresponds to the average drop observed in patients with HIV receiving no treatment. The stabilization of CD4 cells in patients who were administered LDN was also accompanied by disease stabilization. The 55 patients who were non-compliant experienced 25 opportunistic infections, in comparison to the 103 compliant patients who only experienced eight. Survival between the two groups was also significantly different, 13 deaths occurred in the 55 non-compliant patients compared to only one death in the compliant group of 103. At the time of this referenced article (Bihari et al., Sept 1996), patients in Dr. Bihari’s practice had been on LDN for seven to eight years, with no disease progression, no drop in CD4 levels and no evidence of resistance to the beneficial effects of LDN. None of the patients experienced side effects while on LDN.
Dr. Bihari also examined CD4 changes in 19 patients who were on the combination treatment regimen of 3TC (Epivir), azidothymidine (“AZT”), and LDN. The rise in CD4 counts at 6 months in Dr. Bihari’s patients was compared with the rise in CD4 counts reported by an investigator working for Glaxo Smith Kline. In both groups, none of the patients had taken AZT previously; however, Dr. Bihari’s patients simultaneously were treated with LDN, which the Glaxo Smith Kline group did not receive. The patients on LDN had an average baseline CD4 count of 88 while the Glaxo Smith Kline group had an average baseline value of 352. The Glaxo Smith Kline patients experienced an average rise in CD4 of 40 at six months; or an increase of 11.3%. The LDN patients experienced an average rise of 106 CD4’s at 6 months, representing a 128% increase. Of the 19 LDN patients, each patient experienced an increase of at least 30%. In 18 of the 19 LDN patients, a significant increase in energy, appetite and mood was observed. In those LDN patients who were severely underweight, weight gains of ten to 50 pounds were observed in the first two months of treatment.
| 15 | P a g e |
Clinical Trials: Fibromyalgia
LDN has been utilized in a number of trials to investigate its use for the treatment of fibromyalgia. Studies conducted to date in adult patients have typically used an LDN dose of 4.5 mg/day in comparison to placebo.
LDN was assessed for the treatment of fibromyalgia, in a single-blind, crossover trial in which ten women were enrolled. This study utilized the following treatment schedule: Baseline (2 weeks) →Placebo (2 weeks) → LDN 4.5 mg/day (8 weeks) →Washout (2 weeks) LDN reduced fibromyalgia symptoms in the entire cohort, with a greater than 30% reduction of symptoms over placebo. As observed in other studies with LDN, side effects were rare, minor and transient, and included sleep disturbances such as insomnia and vivid dreams.
In a second study, 31 women with fibromyalgia participated in a randomized, double-blind, placebo-controlled, counterbalanced, crossover study. During the active drug phase of the study, participants were administered oral LDN at a dose of 4.5 mg daily. This study showed a benefit with LDN in comparison to placebo for the treatment of fibromyalgia. A significantly greater reduction of baseline pain was observed in those taking LDN in comparison with those taking placebo (28.8% reduction versus 18.0% reduction). LDN was also associated with improved general satisfaction with life and with improved mood. Thirty-two percent (32%, n=9) of participants met the criteria for response (defined by the study as at least a 30% reduction in pain, plus a 30% reduction in fatigue or a 30% improvement in sleep) during LDN therapy, as contrasted with an 11% (n=3) response rate during placebo therapy. Both LDN and placebo were tolerated equally and no serious adverse events were reported.
Clinical Trials: MS
A PILOT TRIAL OF LOW-DOSE NALTREXONE IN PRIMARY PROGRESSIVE MULTIPLE SCLEROSIS was run at the University of Pennsylvania by Dr. Ian Zagon and Pat McLaughlin:
OBJECTIVE: To evaluate the efficacy of 4.5mg nightly naltrexone on the quality of life of multiple sclerosis (MS) patients.
METHOD: A sixth month phase II multicenter-pilot trial with a low dose of the opiate antagonist Naltrexone (LDN) has been carried out in 40 patients with primary progressive multiple sclerosis (PPMS). The primary end points were safety and tolerability. Secondary outcomes were efficacy on spasticity, pain, fatigue, depression, and quality of life. Clinical and biochemical evaluations were serially performed. Protein concentration of beta-endorphins (BE) and mRNA levels and allelic variants of the mu-opioid receptor gene (OPRM1) were analyzed.
RESULTS: Five dropouts and two major adverse events occurred. The remaining adverse events did not interfere with daily living. Neurological disability progressed in only one patient. A significant reduction of spasticity was measured at the end of the trial. BE concentration increased during the trial, but no association was found between OPRM1 variants and improvement of spasticity. The data indicates that LDN is safe and well tolerated in patients with PPMS.
PILOT TRIAL ON LOW-DOSE NALTREXONE AND QUALITY OF LIFE IN MULTIPLE SCLEROSIS:
OBJECTIVE: evaluate the efficacy of 4.5mg nightly naltrexone on the quality of life of multiple sclerosis (MS) patients.
METHODS: This single-center, double-masked, placebo-controlled, crossover study evaluated the efficacy of 8 weeks of treatment with 4.5mg nightly naltrexone (low-dose naltrexone, LDN) on self-reported quality of life of MS patients.
| 16 | P a g e |
RESULTS: Eighty subjects with clinically definite MS were enrolled, and 60 subjects completed the trial. Ten withdrew before completing the first trial period: 8 for personal reasons, 1 for a non-MS-related adverse event, and 1 for perceived benefit. Database management errors occurred in 4 other subjects, and quality of life surveys were incomplete in 6 subjects for unknown reasons. The high rate of subject dropout and data management errors substantially reduced the trial’s statistical power. LDN was well tolerated, and serious adverse events did not occur. LDN was associated with significant improvement on the following mental health quality of life measures: a 3.3-point improvement on the Mental Component Summary score of the Short Form-36 General Health Survey (p = 0.04), a 6-point improvement on the Mental Health Inventory (p < 0.01), a 1.6-point improvement on the Pain Effects Scale (p =. 04), and a 2.4-point improvement on the Perceived Deficits Questionnaire (p = 0.05). LDN significantly improved mental health quality of life indices. Further studies with LDN in MS are warranted.
A PILOT TRIAL OF LOW-DOSE NALTREXONE IN PRIMARY PROGRESSIVE MULTIPLE SCLEROSIS:
OBJECTIVE: To evaluate the efficacy of 4.5mg nightly naltrexone on the quality of life of multiple sclerosis (MS) patients some with relapsing-remitting MS and some with secondary progressive MS.
METHODS: This single-center, placebo-controlled, study evaluated the efficacy of 17 weeks of treatment with 4.5mg nightly naltrexone (low-dose naltrexone, LDN) on self-reported quality of life of MS patients.
RESULTS: The results on LDN’s effect on quality of life (as measured by physical and mental health) was not clearly proven, with no statistically significant differences shown between the LDN-dosed group and the placebo group many have argued the reason for the problem with this trial was the crossover design which have shown in the past not to provide good results when treating with LDN. Data indicates that LDN is safe and well tolerated in patients with PPMS.
RETROSPECTIVE CHART REVIEW OF MS PATIENTS RECEIVING LOW DOSE NALTREXONE (LDN) TO ASSESS SAFETY, TOLERABILITY, AND EFFECT ON FATIGUE:
OBJECTIVE: This study investigated the safety, tolerability, and benefits (fatigue, reduction) of LDN in patients with MS. It reviewed the number of patients who stopped taking LDN and if there were specific reasons for stopping the LDN. The frequency and variety of side effects that were specific to LDN use are reported.
METHODS: A retrospective review was performed on 435 charts of MS patients who were seen in the Penn State Hershey out- patient Multiple Sclerosis Clinic between 1/1/2005 and 5/31/2012. There were 215 MS patients having exposure to LDN during the time of this study. RESULTS: The study confirmed an improvement in quality of life with the use of LDN over a long period of time. In addition to the assessment we review Brain MRIs and Spinal Cord MRIs that were obtained as part of the clinical status of the patient and standard of care. There were essentially no MRIs obtained immediately before LDN was started as would have been ideal in a prospective study. The average number of days of the Brain MRI before treatment with LDN was started was 444 days with a maximum of 4751 days. The average number of days to the latest Brain MRI after LDN was started was 708 with a maximum of 1772 days of 215 patients on LDN 113 MRI scans showed stable, 14 showed improvement, 28 slightly worse. The Slightly Worse MRI series indicated that new but inactive lesion(s) were identified when compared to the pretreatment study MRI. On the quality of life surveys showed 83 patients said they had improved, 92 patients said they were stable and 9 worse. The remainder of the patients did not continue on LDN.
Despite the number of small studies conducted to date, as well as all of the antidotal evidence from patients and doctors that either take LDN for MS or prescribe LDN for MS, Cytocom believes that results are promising, which the Company feels could bode well in larger studies as existing treatments are not every effective.
| 17 | P a g e |
Our Clinical Trial Program for Lodonal™/LDN
The Company held a Type C meeting in January 2018 with the FDA to discuss clinical protocol design and to seek guidance for the clinical development program in the treatment of adult and pediatric patients with Crohn’s disease using a 505(b)(2) pathway to support an NDA. Use of the 505(b)(2) pathway will allow us to seek FDA approval of Lodonal TM without the need to conduct a full development program consisting of both safety and efficacy trials. This will help us to shorten our development pathway and time to NDA submission. We plan to rely on the two drugs using naltrexone that have been approved by the FDA. The first drug approved 50 mg product as the reference listed drug, published literature and historical data and all of the historical data from the 50mg trials and to leverage recent Contrave® approval for safety/PK support at 4mg, 6mg and 8mg with approval granted for Naltrexone at 16mg with Wellbutrin to support the Phase II/III clinical trials and the ultimate filing of the NDA as a 505(b)(2) submission to the FDA. To date, nonclinical pharmacology and toxicology data and clinical safety data has been established from these sources, in addition to multiple Phase I/II studies conducted under our INDs.
To support the NDA filing, we plan to conduct the following pivotal studies:
| ? | Phase IIb, dose-response study/ies in patients with specific autoimmune disease(s); that will rollover into our |
| ? | Phase III, randomized, double-blind study/ies based upon Phase IIB results. |
If NDA approval of Lodonal is granted, in addition to the patents the FDA grants three-year marketing exclusivity granted by law, we expect this product to be protected by patents that extend through at least 2023 for adults 7 year exclusive rights from date of approval for pediatric Crohn’s disease due to orphan indication. During which time it should not be subject to generic substitution. We plan to continue to support the Lodonal program with new patent applications as we obtain data from the clinical evaluation of our new formulation in healthy human subjects and in patients.
If the Phase 2B study of Lodonal is successful, we expect to quickly rollover into the Phase 3 confirmatory study to support product registration.
Based upon the indication chosen, the FDA has stated that a long-term (approximately 1 year) safety study of Lodonal™ will need to be conducted; however, all nonclinical studies to support the safety of the product and NDA submission are complete.
Developing LDN using the 505(b)(2) regulatory pathway lowers the hurdle for FDA approval. Because naltrexone is an FDA-approved product for alcohol or opiate dependence, the FDA’s 505(b)(2) pathway for approval is available and opens the door for us to gain FDA approval of LDN for new diseases. With the opportunity to use previous findings of safety, we intend to use the 505(b)(2) pathway to study and gain approval for our own product.
Patent and Subsidiary Acquisition (from the 10-k)
The Company entered into a share exchange agreement on April 24, 2012 to acquire all of the outstanding shares of TNI BioTech IP, Inc. (“TNI IP”), a biotechnology firm incorporated in Florida and formed to acquire patents related to the treatment of cancer and HIV/AIDS and autoimmune diseases, using Met-enkephalin (“MENK”) and Naltrexone (“LDN”). The goal of TNI IP’s management was to enable mankind and civilization to combat fatal diseases by activating and mobilizing the body’s own immune system using TNI IP’s patented use of MENK. The first patents acquired by TNI IP were acquired from Dr. Nicholas P. Plotnikoff and Professor Fengping Shan in 2012. TNI IP was acquired in exchange for 20,250,000 shares of the Company’s common stock, of which 8,000,000 shares were issued to Dr. Plotnikoff for the acquisition of patents and the remaining 12,250,000 shares were issued to the founders of TNI IP in exchange for all of their right, title and interest in their TNI IP shares. The goodwill arising on the acquisition of TNI BioTech IP, Inc. was valued at $98,000,000 and license agreements arising from the acquisition of TNI IP were valued at $16,006,000.
Intellectual Property
The Company has been developing active forms of immunotherapies through the acquisition of patents, IND Applications, clinical data and all proprietary technical information, know-how, procedures, protocols, methods, prototypes, designs, data and reports which are not readily available to others through public means, and which were owned, generated or developed through experiments or testing by Dr. Plotnikoff, Professor Shan, Dr. Bernard Bihari, Dr. Ian S. Zagon, Dr. Jill Smith, Dr. Patricia J. McLaughlin and Moshe Rogosnitzky.
The Company’s management believes that to be successful, it was imperative to not only acquire all of the intellectual property and patents but also the scientist(s) behind their development. To date, the Company has been able to acquire many of the patents and intellectual property it was seeking, and has also been able to team up with some of the leaders in the field of immunology, experts such as the late Dr. Ronald Herberman (1940-2013), Dr. Angus Dalgleish and Dr. Joseph Fortunak.
Dr. Plotnikoff is the inventor behind a number of patents granted for cancer treatments and an adjunct to patents for autoimmune diseases including: European Patent United Kingdom, Germany, France, Ireland EP 1401471 BI Methods for inducing sustained immune response; Russian Patent Russian Federation patent number 2313364; The Patent Office of the People’s Republic of China, Application No.: 200810165784.8 China Patent CN1015113407 A The Patent Office of the People’s Republic of China ISSN: 1006-2858 CN 21-1349/R; Patent Agencies Government of India Patent, Application number 1627/KOLNP/2003 number 220265 an Enkephalin Peptide Composition; and the US Patent Pending, US Patent Application 10/146.999 e (the “Plotnikoff Patents”). The Patent Cooperation Treaty (“PCT”) enables a U.S. applicant to file a single application, known as “an international application,” in a standardized format in English in the U.S. Receiving Office (the U.S. Patent and Trademark Office) that is acknowledged as a regular national or regional filing in any state or region that is party to the PCT.
The Company entered into a Sale of Technology Agreement with Dr. Nicholas P. Plotnikoff on March 4, 2012, wherein Dr. Plotnikoff agreed to transfer and assign all of his rights, title and interest in the Plotnikoff Patents to the Company. The Company received all the production formulations and technology designs from Dr. Plotnikoff necessary for the manufacturing, formulation, production and protocols of the MENK treatment of cancer and HIV/AIDS. As consideration for entering into the Sale of Technology Agreement, Dr. Plotnikoff received 8,000,000 shares of common stock, a royalty of a single-digit percentage on all sales of MENK and was granted the position of Non-Executive Chairman of the Board of Directors.
In addition to the above patents, we also signed an exclusive licensing agreement for all of the intellectual property developed at Pennsylvania State University by Dr. Ian S. Zagon, Dr. Patricia J. McLaughlin and Dr. Jill P. Smith for the treatment of cancer. The patents cover methods and formulations related to the treatment and prevention of cancers. More specifically, the present inventions describe the use of drugs that interact with opioid receptors (naltrexone, naloxone and the pentapeptide MENK) to inhibit and arrest the growth of cancer. Such efficacy has been discovered to be partially due to the functional manipulation of the zeta opioid receptor through exogenous and endogenous MENK. This receptor has been determined to be present in a variety of cancers, including pancreatic, ovarian, liver, head and neck, and colon cancer. US Patent Numbers 6,737,397, CA 2,557,504, US 20010046968 , US 6737397 , US 6136780 , US 20080015211 , US 20070053838 , US 8003630 ,US 20110123437 , US 7807368 , US 7576180 , US 7517649 , US 20080146512 , US 7122651 , US 20060073565 , US 20050191241 , Patent No 8,003,630.
We also acquired the licensing rights to the patent portfolio and intellectual property developed by Dr. Bernard Bihari relating to treatments with drugs that interact with opioid receptors such as LDN and MENK for a variety of diseases and conditions including malignant lymphoma, chronic lymphocyctic leukemia, Hodgkin’s lymphoma, and non-Hodgkin’s lymphoma, chronic herpes virus infections, and chronic infections due to the Epstein-Barr virus and a treatment method for humans infected with HTLV-III (AIDS) virus including patients clinically diagnosed as suffering from HIV/AIDS and those suffering from ARC. The licensed rights include all reissues or modifications, reexaminations, or other related U.S. patent filings directed to the same subject matter and the use of U.S. Patent Number 6,586,443 , U.S. Patent Number 6,384,044 , U.S. Patent Number 6,288,074 , U.S. Patent Number 5,356,900 , U.S. Patent Number 5,013,739 , U.S. Patent Number 4,888,346 .
Once the Company acquired the above patents, it was then able to sign a licensing agreement to acquire the exclusive patent rights for the intellectual property of the licensors, Dr. Jill Smith and LDN Research Group, LLC, whose members include Dr. Ian S. Zagon, Dr. Patricia J. McLaughlin and Moshe Rogosnitzky. The patents cover methods and formulations for the treatment of the inflammatory and ulcerative diseases of the bowel, using naltrexone in low doses as an opioid antagonist. Endogenous opioids and opioid antagonists at low doses have been shown to play a role in stimulating and rebalancing the immune system and the healing and repair of tissues. US Patent No. 6,136,780, Patent No. US 7879870. The Company then negotiated with Dr. Jill Smith to arrange the transfer of the Orphan Drug Designation for the use of naltrexone for the treatment of pediatric Crohn’s disease with the FDA. Dr. Smith has since transferred the IND to the Company, and the FDA acknowledged that the Company is now the sponsor for this IND. In September 2014, the Company and the licensors jointly agreed to terminate the license agreement, and in place thereof, have the licensors grant a similar license in their patent rights to Cytocom Inc. pursuant to a Patent License Agreement between the licensors, Cytocom Inc. and the Company with substantially similar terms as set forth in the original license agreement. Pursuant to this agreement, the Company issued 1,000,000 shares of its common stock to the licensors and the Company guaranteed the obligations of Cytocom Inc. to the licensors under the agreement.
The Company originally acquired the patents and intellectual property from Dr. Smith and LDN Research Group, LLC because management believed clinical trials involving LDN held great promise for the millions of people worldwide with autoimmune diseases or disorders, central nervous system disorders or those who face cancer. Management also believed it could be the first low-cost, easy to administer therapy with minimal to no side-effects for the treatment of HIV/AIDS, autoimmune diseases and immune disorders, in particular Crohn’s disease, multiple sclerosis, and/or fibromyalgia.
Dr. Nicholas Plotnikoff, Professor Fenping Shan and Noreen Griffin recently filed a Provisional Application for a Utility Patent US Application No. 62/296,759 Method for Inducing a Sustained Immune Response, which was assigned to the Company in March 2016. U.S. Patent and Trademark Office has issued the Official Filing Receipt in connection with this application, and accorded a filing date of February 18, 2016 during its pendency in the USPTO.
...The Company is working with the agencies to obtain local approval for the therapies for each modality that we intend to market for. We believe this will reduce our risk due to The Agreement on Trade Related Aspects of Intellectual Property Rights (“TRIPS”) which is an international agreement administered by the World Trade Organization (“WTO”). TRIPS allows emerging nations to manufacture drugs around existing patents.
https://en.wikipedia.org/wiki/TRIPS_Agreement
Quote from Wikipedia article:
The most visible conflict has been over AIDS drugs in Africa. Despite the role that patents have played in maintaining higher drug costs for public health programs across Africa, this controversy has not led to a revision of TRIPS. Instead, an interpretive statement, the Doha Declaration, was issued in November 2001, which indicated that TRIPS should not prevent states from dealing with public health crises. After Doha, PhRMA, the United States and to a lesser extent other developed nations began working to minimize the effect of the declaration.[8]
A 2003 agreement loosened the domestic market requirement, and allows developing countries to export to other countries where there is a national health problem as long as drugs exported are not part of a commercial or industrial policy.[9] Drugs exported under such a regime may be packaged or colored differently in order to prevent them from prejudicing markets in the developed world.
In 2003, the Bush administration also changed its position, concluding that generic treatments might in fact be a component of an effective strategy to combat HIV. Bush created the PEPFAR program, which received $15 billion from 2003–2007, and was reauthorized in 2008 for $48 billion over the next five years. Despite wavering on the issue of [compulsory licensing], PEPFAR began to distribute generic drugs in 2004-5.
From 3/15/17 publishing...
"Immune Therapeutics CEO Applauds President Trump’s Initiatives for Pharmaceutical Reform
The Company noted that the characteristics of Lodonal, namely its affordability and ability to receive regulatory approval in countries like Kenya, Nigeria and Senegal can provide, the type of solutions that President Trump would like to see implemented by pharmaceutical companies around the globe.
“I applaud President Trump and the measures he is taking to ensure the cost of drugs decrease first by increasing competition and second hopefully reducing the time it takes for a potential drug to hit the market. Immune Therapeutic’s goal since its inception has always been to provide affordable non-toxic therapies and we are proud to have been one of the first biotech companies at the forefront of this movement. We believe our strategy of providing affordable non-toxic sustainable health care therapies for autoimmune diseases as well as HIV and cancer, could propel Immune Therapeutics as an innovative, disruptive, player in the pharmaceutical industry. It is finally time for a change and we are looking forward to supporting President Trump’s and Congress’s initiatives in this arena,” concluded Ms. Griffin."
Recent News from PEPFAR (that should hopefully be related to IMUN, but there is no indication of that in the article)
https://www.pepfar.gov/press/ias2017/index.htm
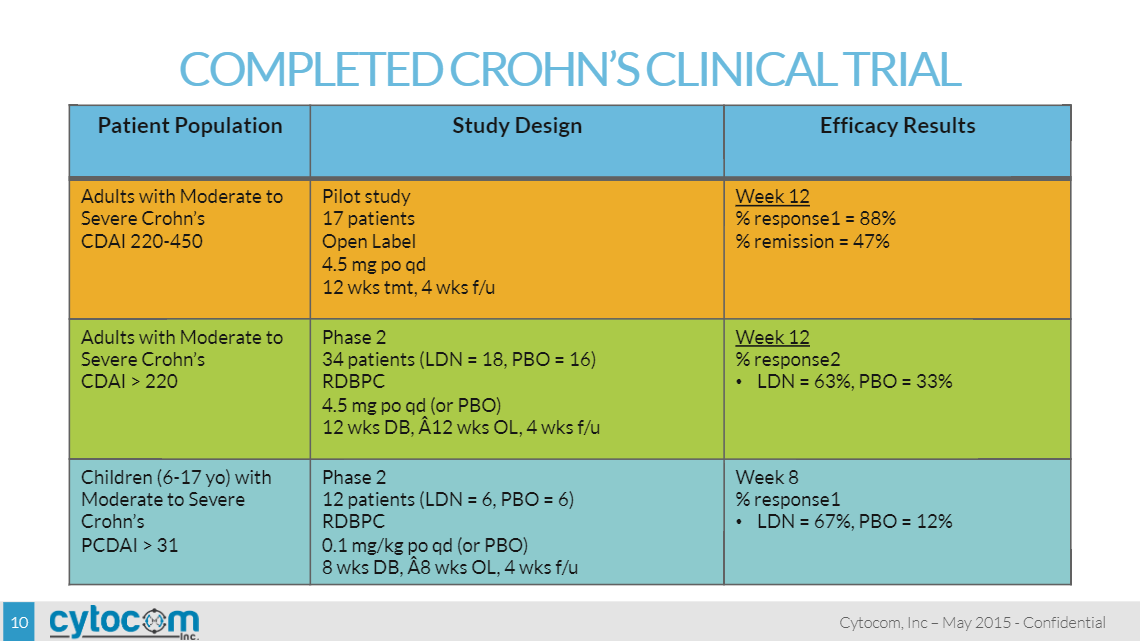
| Volume | |
| Day Range: | |
| Bid Price | |
| Ask Price | |
| Last Trade Time: |
