Register for free to join our community of investors and share your ideas. You will also get access to streaming quotes, interactive charts, trades, portfolio, live options flow and more tools.
LADX one for 100 reverse split:
https://otce.finra.org/otce/dailyList?viewType=Symbol%2FName%20Changes
News release out. Reverse split coming next week.
CytRx Corp., CYTR, changed to LadRx Corp., LADX:
https://otce.finra.org/otce/dailyList?viewType=Symbol%2FName%20Changes
Bye bye. Another retail crushing rs. Rinse and repeat. Next CEO!!!
Approval of an amendment to our Restated Certificate of Incorporation (“Certificate of Incorporation”) to effect, at the discretion of the Board but prior to the one-year anniversary of the date on which the reverse stock split is approved by the Company’s stockholders at the Annual Meeting, a reverse stock split of all of the outstanding shares of our Common Stock at a ratio in the range of 1-for-2 to 1-for-100, with such ratio to be determined by the Board in its discretion and included in a public announcement (the “Reverse Stock Split Proposal”)
Do we think Kriegsman also snowed Snowdy?
It was always a Scam
Starting to truly believe it’s a scam!! I’ve lost thousands, believing in this crooked BS
IS THIS STOCK A SCAM OR WHAT??? I WANT THE TRUTH!!!
Bravo!!! So long Kriegsman!
CytRx Corporation (OTCQB:CYTR) ("CytRx" or the "Company"), a specialized biopharmaceutical company focused on research and development in oncology and neurodegenerative diseases, today announced that it has appointed Dr. Stephen Snowdy to the role of Chief Executive Officer, effective January 10, 2022. Dr. Louis Ignarro succeeded Steven A. Kriegsman as Chairman of the Board of Directors (the "Board") on January 3, 2022.
Dr. Snowdy is a scientist, serial entrepreneur and medical venture capitalist with two decades of experience in life science investing and executive management.
Dilute, dilute, dilute. Kreigsman needs his salary and rent. Rose gardens arn't cheap.
CYTR...92...Bullish Descent Block Reversal Pattern to the Upside...Open Gap to fill in the $1.50/$2.50 range...
Chart...https://schrts.co/yuNqDEVZ ...
And, all we get is a vague PR on the FDA issues with the trial. For all we know the trial never stood a chance from the very beginning. Just more research and OTC salaries.
LOL...This never showed a profit, cash incinerator, CYTR made him rich at the retail investors expense. This company isn't worth $.02, but, as long as retail investors don't mind losing their shirt, the show will go on and the C suite crowd will further enrich themselves.....
FDA doesn't like their arimoclomol for Nose-Pick disease.
CYTR...Filled some at .88...:party:
CYTR...$1.08...Back in on the dip and looking for this Open Gap to eventually fill...imo...we shall see...
Took quick profit on my $1.50 range alert in the $2 range but the move saw a $4.00 range move...
Dip based on this News...https://www.otcmarkets.com/stock/CYTR/news/CytRx-Notes-Orphazymes-Regulatory-Update-from-the-FDA-on-Arimoclomol-for-Niemann-Pick-Disease-Type-C?id=307976
Chart...https://schrts.co/WWgNhEPY ...
It'll be busy today....
Those royalties about to be kickin in nicely
And what’s that??
What's happening?!?!? Whatever it is I like it!
so quiet here considering whats about to happen!
CYTR...$1.52...Bullish Harami forming on the daily...:partying_face:
CYTR...$1.81...:party:
[9:41 AM]
georgie18 — 05/21/2021
CYTR...$1.65...Back in on the 200ma break...:party:
Revenues, 32 MILLION shares, and Audited financials give me some of these shares please.
That's a logical guess. The chart says some knew something months ago as it's been accumulation ever since: buy, let it relax, buy, let it relax,...
I saw the same on CRIS last Summer, but it was for a different reason.
I have always thought they end up in NK
Yeah, I think that last 8-K says we're getting closer to M/A.
lol. They wont let me back in...
Morning,
Good trade!
I'm still holding from 12/14/20. I was tempted as well, but the last 8-K told me to wait/hold...
Sold on shake... now I need to chase.
Actually the word "parachute" is too harsh, more like "provision".
That the bod would allow the CFO to issue this provision in an 8-K, instead of just burying it in the next March 10-K (the 10-Qs are in 5/xx, 8/xx, 11/xx) implies a higher probability of an imminent M/A.
Got ya, It all became a little clearer, after I read the eighth line in the 8k a couple of times. >>>employment is terminated without “cause” following a “change in control” (as defined in his employment agreement))<<<<
It wasn't a criticism, merely a precursor for an expected occurrence...
a buy-out.
I say tomato and some say potato, either way it is a pittance and CYTR has a bushel full of CA$H to pay it.
Yes, and:
"...agree in Mr. Caloz’s employment agreement that if we do not offer to renew or extend the employment agreement we will continue to pay Mr. Caloz’s annual salary thereunder during the period commencing on expiration of the employment and ending on June 30, 2022 or the date he is employed by another employer, whichever is earlier."
Mr. Caloz has entered into a new employment agreement which would tend to predict another years employment with CYTR.
>>>On January 5, 2021, CytRx Corporation (“the Company”) entered into a new Employment Agreement, effective January 1, 2021, with John Y. Caloz, under which we agree to continue to employ Mr. Caloz as our Chief Financial Officer through December 31, 2021<<<
8-K: Mr. Caloz has a new parachute, and is predicting he might not be the CFO in the near future?
For the newly curious about CYTR:
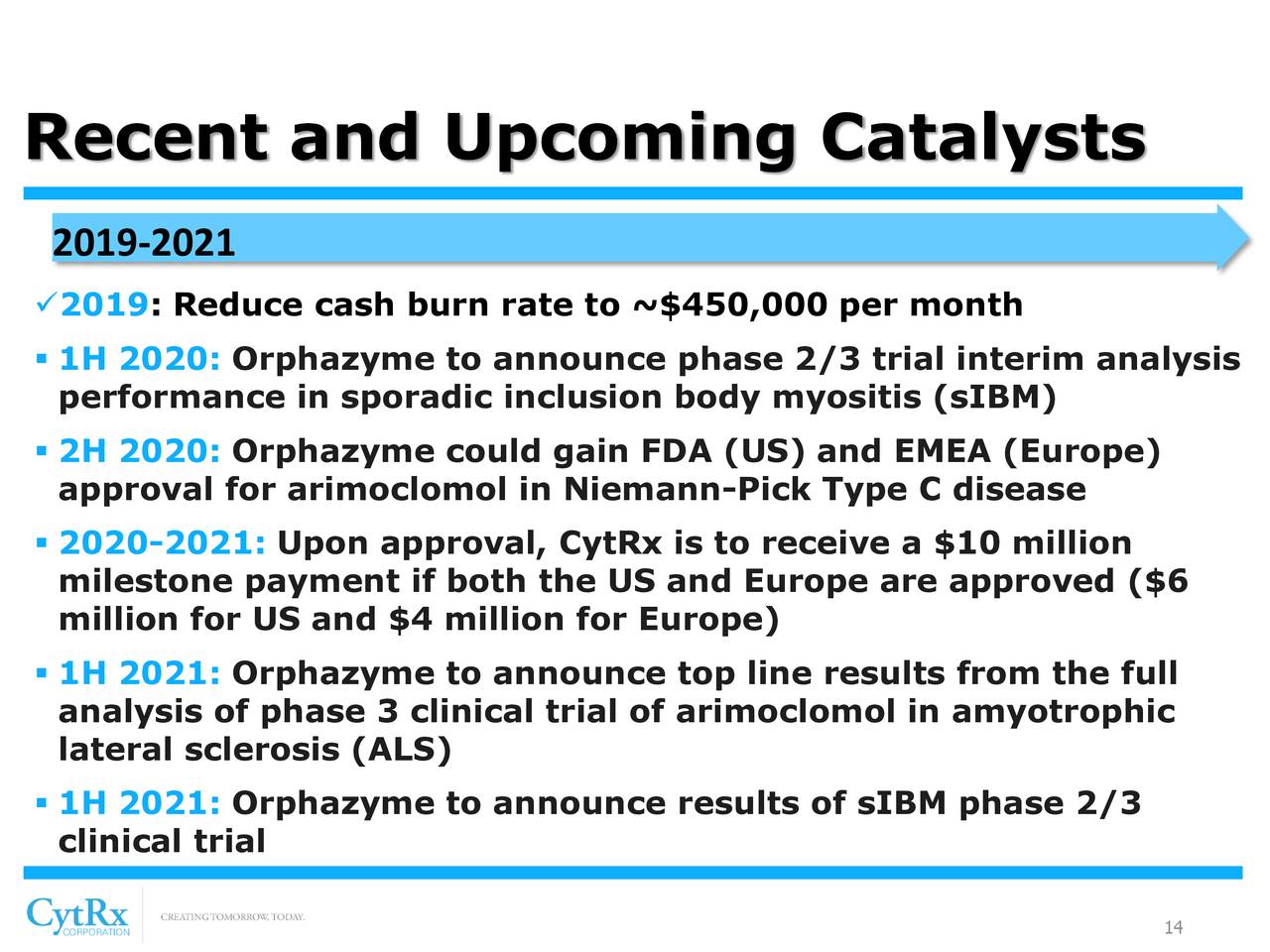
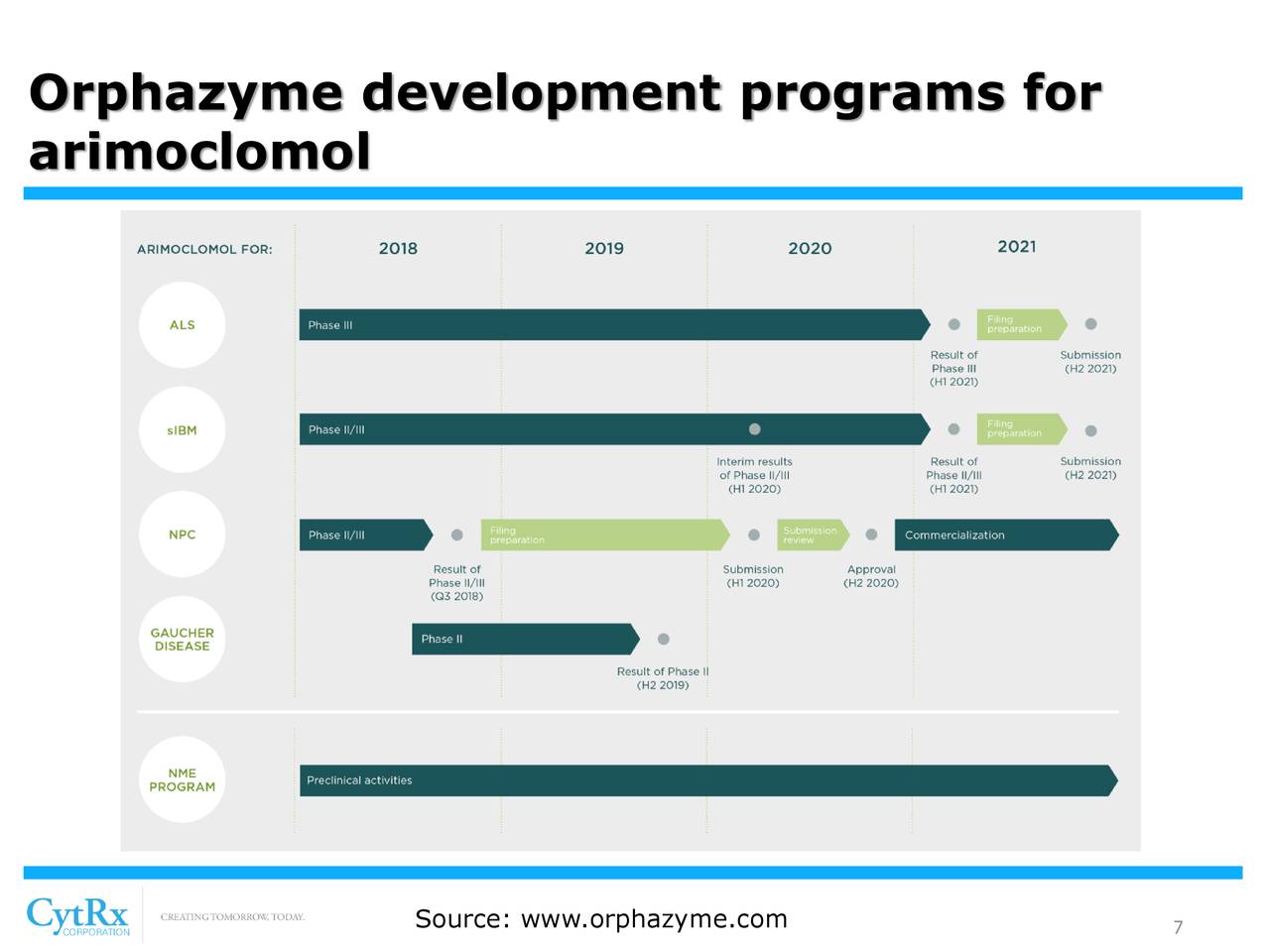
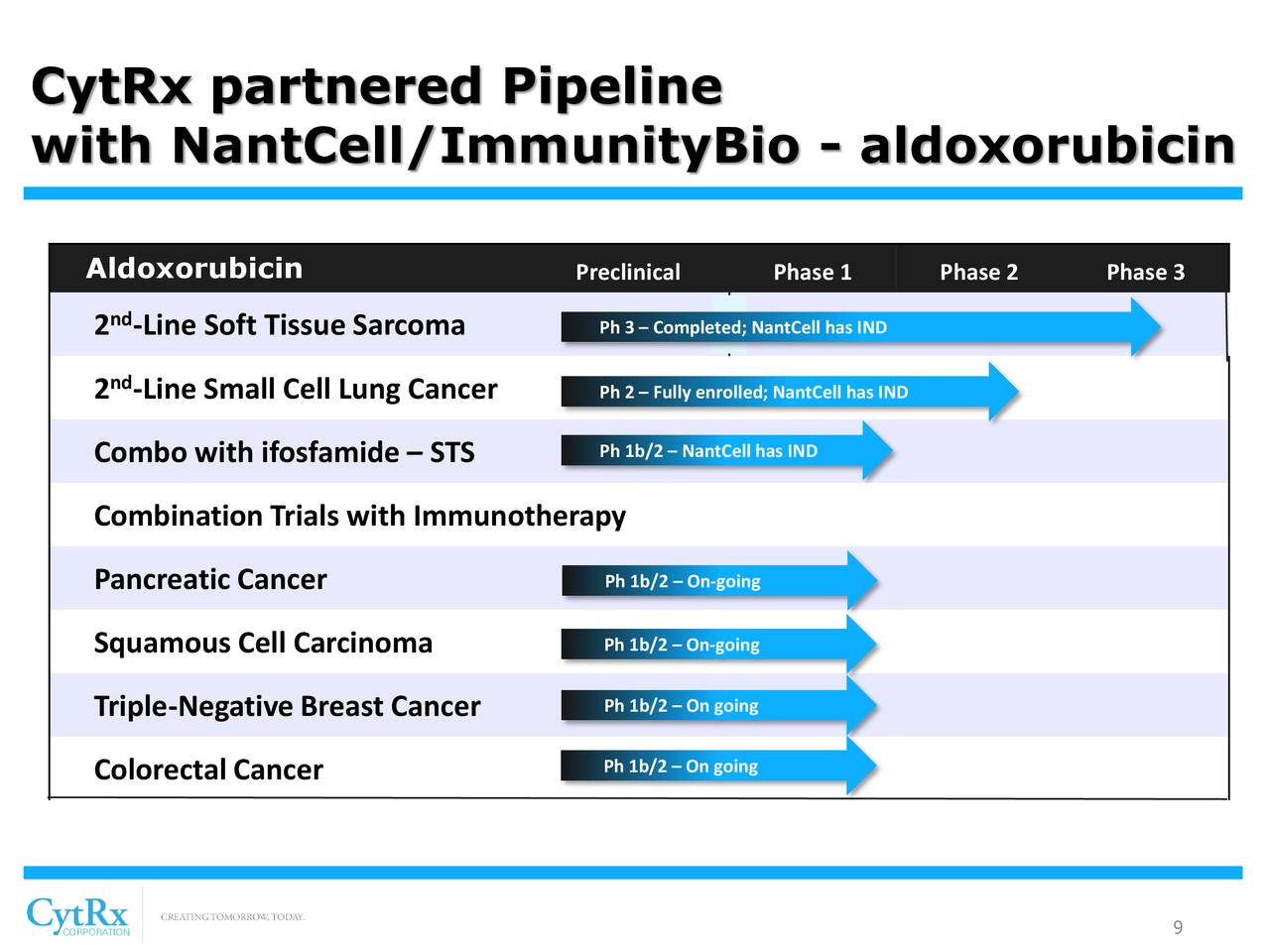
CytRx Corporation (OTCQB: CYTR) is a biopharmaceutical company with expertise in discovering and developing new therapeutics principally to treat patients with cancer and neurodegenerative diseases. CytRx's most advanced drug conjugate, aldoxorubicin, is an improved version of the widely used anti-cancer drug doxorubicin and has been outlicensed to ImmunityBio, Inc. In addition, CytRx's other drug candidate, arimoclomol, was sold to Orphazyme (Nasdaq: ORPH) Orphazyme A/S (Nasdaq Copenhagen exchange: ORPHA.CO) in exchange for milestone payments and royalties. Orphazyme is testing arimoclomol in four indications including amyotrophic lateral sclerosis (ALS), Niemann-Pick disease Type C (NPC), Gaucher disease and sporadic Inclusion Body Myositis (sIBM). CytRx Corporation’s website is www.cytrx.com.
Bouncing back quickly...
Enjoy...
|
Followers
|
208
|
Posters
|
|
|
Posts (Today)
|
0
|
Posts (Total)
|
7747
|
|
Created
|
01/22/05
|
Type
|
Free
|
| Moderators | |||
| Volume | |
| Day Range: | |
| Bid Price | |
| Ask Price | |
| Last Trade Time: |
