Register for free to join our community of investors and share your ideas. You will also get access to streaming quotes, interactive charts, trades, portfolio, live options flow and more tools.
MPME registration revoked:
https://www.sec.gov/litigation/opinions/2020/34-89800.pdf
Does this still hold?
81vette !!!ALOT ARE IGNORING THIS GEM, $$$ MPME COULD HAVE A BIG BOOM NEXT WEEK..BIG NEWS COULD BE IMMINENT...DOLLARS COULD BE FAST..
MPME SEC Suspension for severely delinquent Financials:
https://www.sec.gov/litigation/suspensions/2019/34-86647.pdf
Order:
https://www.sec.gov/litigation/suspensions/2019/34-86647-o.pdf
Admin. Proceeding:
https://www.sec.gov/litigation/admin/2019/34-86646.pdf
81vette !!!ALOT ARE IGNORING THIS GEM, $$$ MPME COULD HAVE A BIG BOOM NEXT WEEK..BIG NEWS COULD BE IMMINENT...DOLLARS COULD BE FAST..
ss updated 10 days ago, should be soon we find out why, this is the lowest float I have ever seen. could be anything coming good or bad ,if its good ,the run will be epic !! if its bad ,not many in and its already at a low so not going to drop epic lol
81vette ..MPME $$$ SOMETHING BIG GOING ON HERE BEHIND THE SCENES...WITH BIG NEWS THIS COULD SEE $1.00+ FAST..IM WILL TRY TO LOAD.
share structure updated ,transfer agent verified ,some kind of change is coming , ss update is required for many things,merger/sale/custodianship,new change ect.
What do you think might be happening here?
if its custodianship ,easy 10x with this float
MPME $$$ LOOKS DIRT CHEAP, ABOUT TO GO INSANE..WHAT AN UNKOWN GEM.
MPME the new CEO Sophia Sun is shown in the following PR
Profectus and Medisun Launch ProMed BioSciences to Develop and Commercialize Vaccines to Prevent and Treat Infectious Diseases and Cancer in Asian Countries
https://www.prnewswire.com/news-releases/profectus-and-medisun-launch-promed-biosciences-to-develop-and-commercialize-vaccines-to-prevent-and-treat-infectious-diseases-and-cancer-in-asian-countries-300650163.html
I am holding all mine. I will wait and see West Oak Capital's new filing later this month.
Somebody selling out today. Unfortunately they still didn't commence filings and there is a risk holding it as long as they are severly delinquent.
Nice little volume bump today! This ultra low floater could really move with a few more volume days like today!
MPME Excellent play!!!
MPME 0.11 x 0.14 PM volume; watch for a run back to 0.2 today
MPME
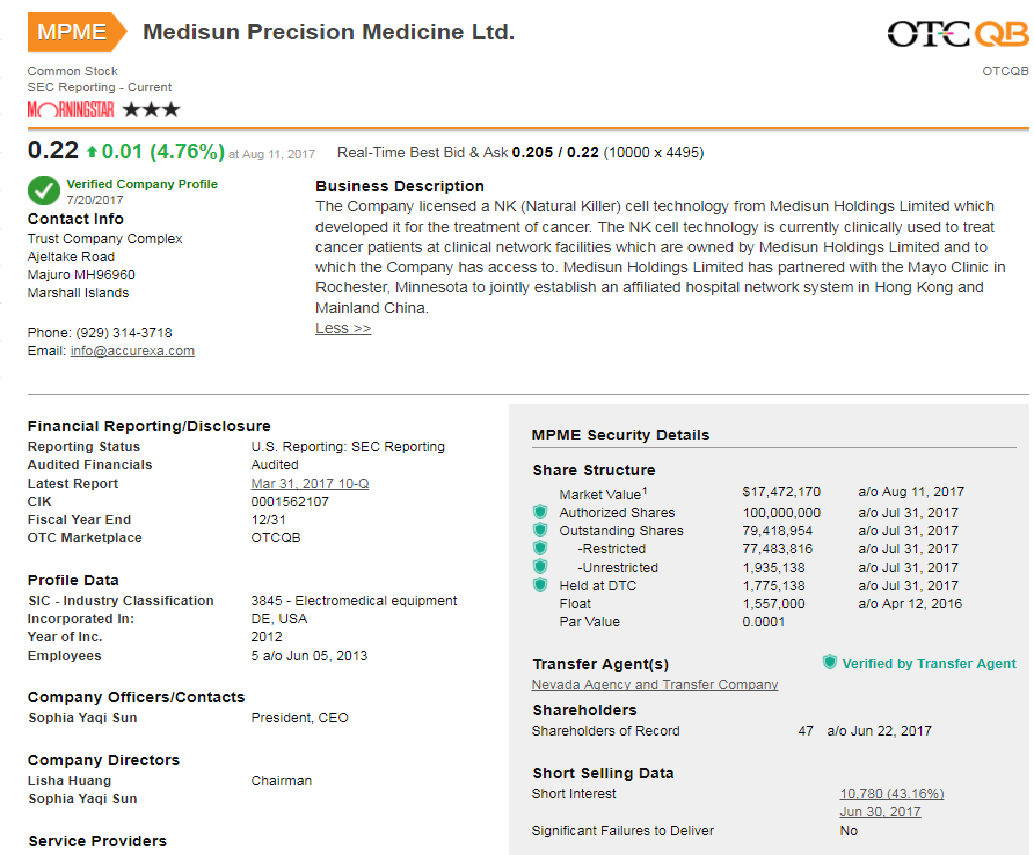
https://www.otcmarkets.com/stock/MPME/profile
The Company licensed a NK (Natural Killer) cell technology from Medisun Holdings Limited which developed it for the treatment of cancer. The NK cell technology is currently clinically used to treat cancer patients at clinical network facilities which are owned by Medisun Holdings Limited and to which the Company has access to. Medisun Holdings Limited has partnered with the Mayo Clinic in Rochester, Minnesota to jointly establish an affiliated hospital network system in Hong Kong and Mainland China.
http://www.medisun.hk/product?information_id=95
5 NK CELLS
There are "natural killers" in the human body that can fight cancer.
Natural killer cells (NK cells), which are ubiquitous in the human body, are one of the most potent immune cells in the human body. After being matured in the bone marrow, they mainly exist in the peripheral blood, liver, and peritoneal cavity. In the placenta, when they encounter cancerous cells, they kill them, and they also stimulate the body to produce anti-tumor immune responses. So it is called “the first line of defense against cancer” in the medical community.
?
NK cells have a strong killing effect on cancer cells
It is understood that the human body usually produces thousands of cancer cells every day. NK cells have a strong killing effect on cancer cells. Once cancer cells are found, they will immediately combine with them to destroy cancer cells and kill them. It is highly efficient and natural. Hunting Cancer Expert."
In fact, the amount of NK cell activity is reduced by certain types of stress. When the body is unable to fully adapt to the environment, physical function declines, lack of sleep, excessive work, unsatisfactory feelings, lack of exercise, and nutritional deficiencies are all contributing to physical stress. Even temporary stress can lead to an acute decrease in NK cell activity, but with proper adjustment and rest, NK cell activity can be elevated in the body by resting and eating appropriate foods. However, as we continue to be under pressure and dissonance in our living environment, the weakening of the immune system may lead to cancer or other chronic diseases.
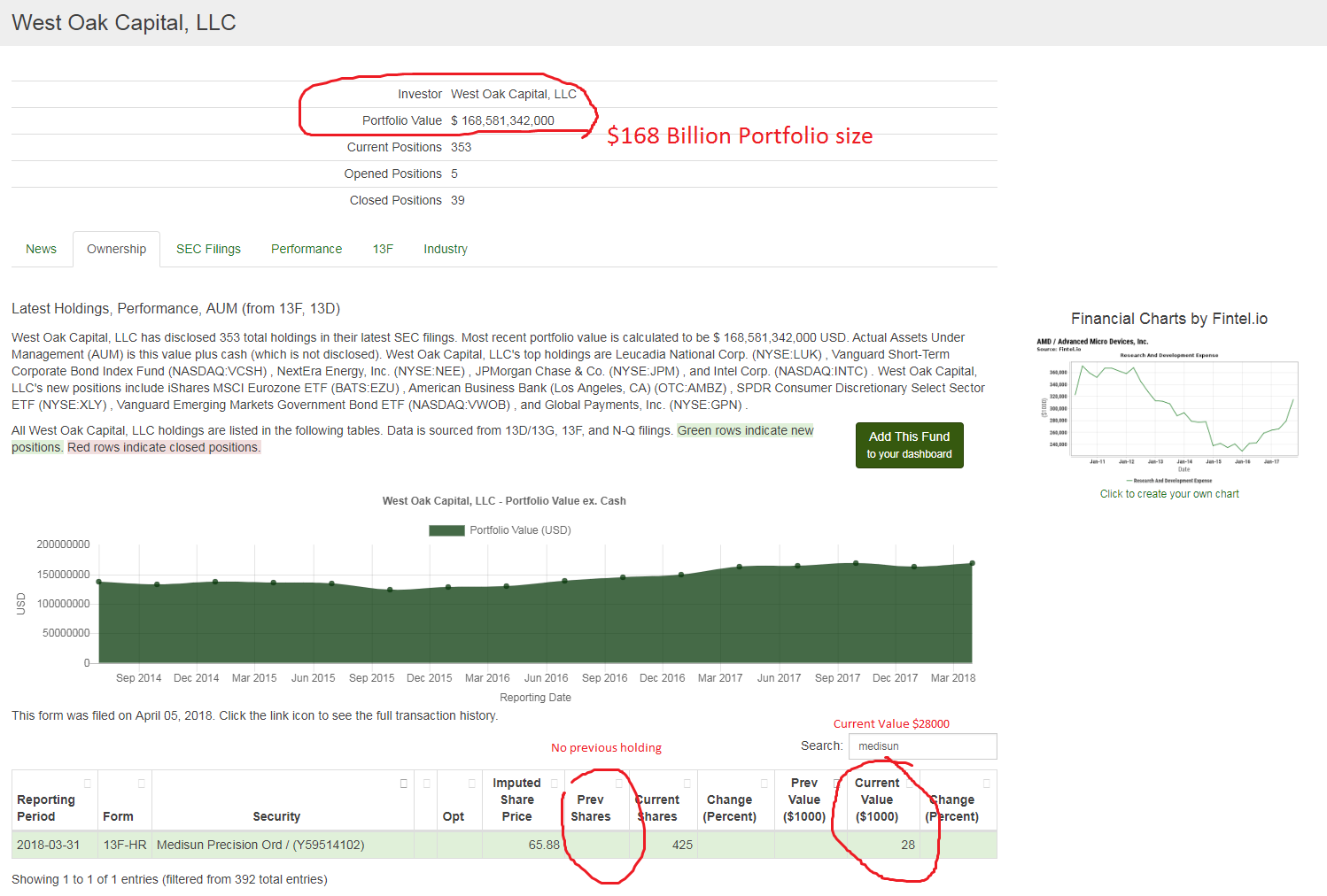
float should be around 1.5M shares before 425K shares bought by west Oak Capital LLC
MPME Solid play n super thin.
this one has got into silence after the reverse merger. that's why I am surprised to see a Billion $$ investor firm bought in
MPME it is rare for a billion $ institutional investor showing up on PINKSHEET
MPME DD tells it all. Huge move coming here. 1/3 float locked from institutional investor West Oak Capital LLC
MPME looks like the buying spike in Jan 2018 was from institutional investor West Oak Capital LLC
MPME institutional investor West Oak Capital LLC bought 425K shares from Jan 2018 to March 31 2018. Form 13F showing the new position
West Oak Capital LLC Portfolio value $168 Billion!
https://fintel.io/i/west-oak-capital-llc
search Medisun in the search box for quick search
Oh yes, it is about time. GLTA
noticed that. Watching
Back down to 07, amazing POS this one.
Unfortunately, yes, need patience
MAXM at the ask again and basically no action lately. Back to waiting.
sabb management got MPME shares at $1.2+ from previous filings. I will hold mine for $1 at least
Very good if he is gone for good now. Let's see something here.
MPME MAXM gone on ask 0.1 X 0.1176
Weird, I got the notification that there is a new SC13 filed but it seems they sent me the alert one week late for the january 4th filing. Never seen this before. So in this case nevermind.
filing was on Jan 4 2018
New SC13G filing.
Any update from company will wake her up.
Traderfan,
He doesn't have to have many followers to move the stock since it really doesn't trade much volume. I've followed him for several months and he has had some big winners and I have seen smaller stocks move on his recommendations. Even though today is just because of the big spread, it's still nice to see an up day.
Ah I see. Could that be the readon for the sudden continued selling every single day? Does the guy have so many followers?
MPME, any update from the company?
The author recommended the sale of ACXA which is the recommendation of the sale of MPME. The readers that follow this author are selling on his recommendation.
Not sure what that link has to do with this stock?
It likely had something to do with this:
https://seekingalpha.com/instablog/47797823-melikemsmallstocks/5057063-knocked-horse-get-back-mantra-biotech-investor-look-back-last-12-months
Not uncommon for stock to move a bit when his pieces come out.
|
Followers
|
9
|
Posters
|
|
|
Posts (Today)
|
0
|
Posts (Total)
|
1210
|
|
Created
|
06/27/14
|
Type
|
Free
|
| Moderators | |||


| Volume | |
| Day Range: | |
| Bid Price | |
| Ask Price | |
| Last Trade Time: |
