Friday, September 25, 2020 12:17:29 PM
( From amstocks82 on 2/20/2020, I did not repeat the post in February because of my own poor health at the time. I thought this would be a good time to do so.
Another February post from Amstocks is repeated at the end.)
Alzheimer’s disease is characterized by a gradual decline of the ability to reason and remember. Alzheimer’s disease can be diagnosed based on the presence of:
1) Amyloid Beta Plaques
2) Neurofibrillary Tangles (NFTs).
Amyloid beta plaques are characterized by clumps of amyloid beta mixed with degenerated brain cells and structures. These are also called neuritic or senile plaques.
Neurofibrillary tangles are formed by a clumping of a degraded form of the “tau protein”.
Alzheimer’s disease leads to a gradual decline of the ability to reason and remember due to the progressive damage to the brain structures and neural structures (neurons). (Neurons and neural structures will be discussed more fully below.)
From Alzheimer Org, https://www.alz.org/ :
Alzheimer's disease is the sixth-leading cause of death in the United States.
An estimated 5.8 million Americans of all ages are living with Alzheimer's dementia in 2019. This number includes an estimated 5.6 million people age 65 and older and approximately 200,000 individuals under age 65 who have younger-onset Alzheimer's.
One in 10 people age 65 and older (10 percent) has Alzheimer's dementia.
A Mini-Mental State Examination (MMSE) is often used to categorize the progression of Alzheimer’s disease.
The MMSE test has a maximum of 30 (30 being perfect). A MMSE score of 24 or greater indicates normal cognition. A score of 19 to 23 indicates mild impairment. A score of 10-18 is moderate impairment. And any score below 10 indicates a severe impairment.
The average length of time someone lives who gets Alzheimer's disease is ~4.5 years if diagnosed after age 70. The average MMSE for people diagnosed with Alzheimer’s disease is around 24 to 26.
Neurons are made up of three main parts: The cell body or soma, dendrites and the axon. Dendrites are extensions of the neuron that receive signals and conduct them toward the cell body. The axon is an extension of the neuron that conducts signals away from the cell body to other cells. The length of the axon may be 1,000 to even 10,000 times longer than the width of the cell body.
Below are three diagrams of neurons highlighting different aspects of neuron biology.
Diagram 1, Neuron picture:
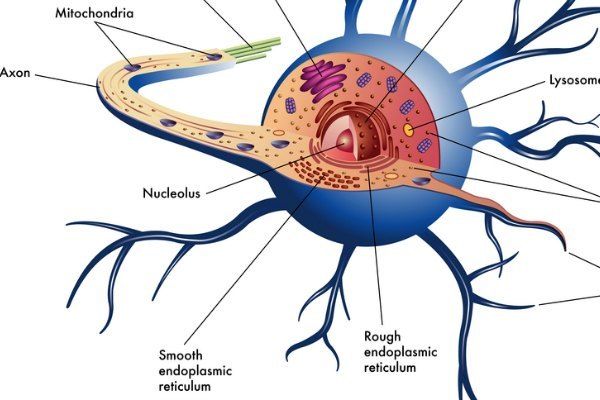
Diagram 2, Neuron picture:

A typical brain has over 100 billion neurons. Each individual neuron may network or form physical connections to several thousand other neurons (it is estimated that the number of connections can be up to 10,000). The number of connections in the brain could be on the order of 100 trillion to 1,000 trillion connections via a specialized connection called a synapse. The synaptic connection is typically via an axon to the dendrite of another neuron. However, some connections form between axon to axon or dendrite to dendrite.
Diagram 3 of Neuron picture:

Electro-chemical interactions occur between neurons when information is transmitted within the brain. Physically, all cells work by chemically manipulating molecules to form various types of organic molecules used to build structures or form useful compounds such as proteins or fat. They also break down or alter molecules in order to release or store chemical, heat or even electrical energy. Electrochemical transmission of information between different neurons is accomplished by various neurotransmitters.
Neurotransmitters are stored and released at the neuron’s axon terminal. When activated, a neurotransmitter is released at the axon terminal and it moves across the synaptic cleft between neurons to the dendrite of the receiving neuron where the transmitted neurotransmitter binds. This can cause an electrical signal to be generated within the receiving axon transmitting information within the neuron potentially to one or more other neurons depending on the signal.
Neurotransmitters are stored in the axon in organelles called "vesicles". There are several vesicles at the axon. They contain various neurotransmitters such as acetycholine, dopamine, histamine, serononin, melatonin, adrenalin and finally, the most common transmitters are glutamate and GABA. Neurons are very energy "intensive" given that a typical neuron sends an electrical or electro-chemical pulse to another neuron between 5 and 50 times per second.
The current view of Alzheimer’s disease is that there are 5 different issues most of which are interrelated:
1) Alzheimer’s patients have “amyloid plaques” located within the brain tissue. These plaques are caused by “Amyloid beta” peptides.
Diagram 4 – neurofibrillary tangles

Alzheimer’s disease, Amyloid Precursor Protein and Amyloid Beta
Amyloid beta (Aß) in humans is usually defined as peptides with 36 to 43 amino acids that have been found to be the main component of the amyloid plaques found in the brains of Alzheimer patients. Proteins are also composed of amino acids. Proteins are distinguished from peptides based on size. In the older definition, a peptide usually has fewer than 50 amino acids and proteins have more than 50. Another common definition is that a protein is a stable and complete biological molecule. Usually a protein has a 3-dimensional structure. A peptide is a subset of a protein and therefore, not a complete form of the functional biological molecule and usually does not have a 3-dimensional structure.
Amyloid beta (Aß) is formed when a protein called Amyloid Precursor Protein (APP) is “divided” into smaller sections. APP (Amyloid Precursor Protein) was identified due to research into amyloid beta (Aß).
Amyloid Precursor Protein or APP
Amyloid Precursor Protein or APP is found in in several organs including the brain and spinal column. APP is an ancient protein (meaning probably evolved hundreds of millions of years ago), and like many ancient proteins, it likely has multiple functions – many of which people are now speculating about. For example, as regards Alzheimer’s disease, it is believed APP is used in the neurons to both form synaptic junctions and to repair them.
Some other functions of APP have been found. APP has some impact on cell signaling. APP has an effect on neuronal transport of iron and in transport of some molecules to synapses that may be located quite a way from the cell center. These are only the known or suspected functions of APP. There are studies that indicated that APP may help cells attack to one another and that in the brain, it could help neurons in the brain and central nervous system migrate to the correct location during fetal development. There are already a few other possibilities that are speculated upon and likely many others waiting to be discovered.
APP as befits ancient proteins is “highly conserved”. In nature, if something is “highly conserved”, it means that it is present across many lifeforms and animals. This in turn means that whatever is conserved is not only critical but that nothing better has been discovered in the evolutionary process. It must be critical because animals that have mutations that don't pass on the "highly conserved" trait are not currently around. Any animal that evolves without APP quickly dies out.
APP breaks down into smaller molecules called Amyloid Beta (Aß). APP can be broken down by the body in various ways. The most common Aß form in healthy cells is for it to be broken down into a peptide of length 40 (Aß40). This is done in an “organelle” of the cell called the trans-Golgi network. Another more toxic form is Aß42. This is mainly formed on the endoplasmic reticulum. Several other Aß forms are also created in various ways in the cells usually in lesser amounts than Aß40 or Aß42. Some of them result in Aß that is fairly “toxic” to cells. Larger amounts of Aß42 tends to be found in diseased cells.
In healthy persons, the various forms of Aß are usually broken down further by various enzymes that specifically target Aß. These enzymes are typically called amyloid degrading enzymes. In healthy brains, excess amounts of Aß do not continue to accumulate and Aß is not continually excreted from the cells in large amounts such that it forms Aß plaques between cells.
Aß is often linked with the term “misfolded proteins”. In reality, Aß is not a protein. Nor does the typical “misfolded” protein description strictly apply. Aß is a fragment of a protein which is too small to be called a protein. Aß is a “peptide”. Excess amounts of certain forms of Aß tend to aggregate together and can do so in “forms” which appear to poison cells. In some of the Aß aggregations, the peptides are folded in ways that make it even more toxic. Another reason that the term “misfolded proteins” does not apply is that after ribosomes (a cell structure) build proteins, they are “folded” in parts of the endoplasmic reticulum so that the protein can perform its biological function. In Aß, the “folding” problem happens after the protein APP is “cleaved” into smaller sections forming Aß.
There are several other “mysteries” surrounding Aß. The various peptide forms of Aß may have cellular functions. The reason to think this is because Aß is formed by “cleaving” APP in very specific ways by types of enzymes that are also highly conserved. In other words, the enzymes that cut up or breaks apart the APP protein into smaller Aß peptides are something that is widely present in similar form in a wide range of animals and life forms. Thus, the structure of the enzymes that cuts APP must be critical. And it would follow, that it is cutting them into various types of Aß with varying lengths for specific biological functions even if we don’t know what all of those functions may be. Lending some additional insight into why this might be so is a recent study on one function of Aß working as an antibiotic:
“A new study from Massachusetts General Hospital (MGH) investigators provides additional evidence that amyloid-beta protein -- which is deposited in the form of beta-amyloid plaques in the brains of patients with Alzheimer's disease -- is a normal part of the innate immune system, the body's first-line defense against infection. Their study published in Science Translational Medicine finds that expression of human amyloid-beta (A-beta or Aß) was protective against potentially lethal infections in mice, in roundworms and in cultured human brain cells. The findings may lead to potential new therapeutic strategies and suggest limitations to therapies designed to eliminate amyloid plaques from patient's brains.” https://www.sciencedaily.com/releases/2016/05/160525161351.htm
Why is this important? I believe that the formation of Aß is something “regulated” by the cells and by various organisms from humans to roundworms. Given how conserved the function of the enzymes are for cutting apart APP into small peptides, it seems logical that not only does APP have many roles in the cell, but that Aß must also. The recent study that Aß helps fight certain disease leads credence to this.
Therefore it is possible that the accumulation of Aß may be a consequence of cellular misregulation or due to inflammation processes. If a cell is “inflamed”, it may be undergoing an immune reaction where the cell is reacting to what it believes is an infection or attack. A younger healthier person has more robust regulation of cellular mechanisms due to producing higher concentrations of regulatory, hormones, having better circulation, having better metabolic regulation and better overall health. Therefore, the brains of younger people recover from such inflammation processes while the brains of older people sometime don't. Instead, the inflammation triggers other problems causing the cascade of issues ultimately leading to full blown Alzheimer’s disease.
Drugs that target Aß have not been effective because the misregulation of cellular mechanisms is not corrected by drugs that target Aß. And in fact, targeting Aß may make the misregulation issues worse. Just as too much Aß is a problem, too little may also be a problem.
Anavex2-73 does not target Aß directly. Rather, the impact is to signal the cell that it is under “stress” and should change cellular behavior in ways to reduce the stress and help the cell return to homeostasis.
The way it does this is mainly as a Sigma-1 Receptor (S1R) agonist and mixed muscarinic receptor agonist. The most widely known of those is the S1R which is what is called a receptor chaperone. For example, one of several functions is to modulate calcium signaling in the cell. Other changes in the receptor can signal the cell to die via programmed cell death (apoptosis) or signal it to try to recover and fight disease processes leading towards homeostasis.
See Anavex presentation slide diagram, “S1R = Sigma-1 Receptor”
Diagram 5 S1R (Anavex presentation):

To make a comparison, for a person with diabetes, insulin brings the blood sugar levels back to healthy homeostatic levels which allow diabetics to function normally. For Alzheimer's patients, Anavex2-73 may help to similarly bring cellular metabolic processes back towards homeostasis which is what the lab results and human trial results suggest.
APP is divided by enzymes that are called beta secretase and gamma secretase. The enzymes exist for the sole purpose of dividing or cleaving APP. Those enzymes cleave APP in ways that yield amyloid beta or Aß.
Aß molecules can aggregate to form flexible soluble oligomers (or small biological structures) which may exist in several forms. An oligomer usually refers to a macromolecular complex formed by non-covalent bonding of a few macromolecules like proteins or nucleic acids. This oligomer or macromolecular structure has a complex architecture in 3 dimensions and contain various folds and curves.
2) Alzheimer’s patients have neurofibrillary “tangles” in their brain tissue.
Neurofibrillary tangles are associated with tau hyperphosphorylation. Phosphorylation is when a phosphoryl group attaches to another molecule. Through phosphorylation, and dephosphorization, proteins (enzymes) are activated and deactivated. If something is hyperphosphoylated, it can mean there is some loss of function of the protein.
Diagram 6 Microtubule Subunits Fall Apart:

Tau protein is a major microtubule-associated protein (MAP) in a human brain cell or neuron. One of tau's main functions is to modulate the stability of axonal microtubules.
Axonal Microtubules
What are Axonal Microtubules? They are major architectural (structural) elements within the neuron which allow it to maintain its shape and help to convey and aggregate materials at specific locations within the cell or neuron. Neurons have an axon and dendrites that spread out from the neuron cell body. The axon can be 1,000 to 10,000 times the length of the cell body. Given the length of the axon especially, it requires structural elements as well as some way to convey “materials” the length of the axon.
Microtubules in addition to being structural elements function like railways that convey cargo within the cell or neuron through the axon and dendrites of the neuron. The “cargo” of molecules or organelles that move along the microtubules are everything needed to maintain, provide fuel (glucose) and repair the neuron cell body, axon and dendrites. The microtubule “cargo” is also needed for such things as creating new “associations” (memories) and operating the synaptic junction. Other microtubules work in the opposite direction carrying materials in the reverse direction back from the axon or dendrite to the main neuron cell body. Microtubules are essential for moving mitochondria to where they are needed such as areas where the cell needs energy. Mitochondria move “long” distances such as within an axon or within dendrites along microtubules.
There are molecules in the neuron that regulate the activity of microtubules and how they are formed. Microtubule-associated (tau) protein (MAP) that undergoes hyperphosphorylation becomes toxic to the neuron and can lead to the death of the neuron as well as causing the neuron to operate in a less than optimum fashion. The neuron has a defense mechanism against this toxic protein. That defense mechanism degrades the hyperphosphorylated tau further into a non-toxic but inert material. If the neuron were operating efficiently, this material would get removed from the neuron over time. But if it is not working well, the inert material accumulates in the neuron and also in extracellular spaces. If sufficient of this material accumulates, it gets in the way of other cellular mechanisms effectively "clogging up" the biochemical processes of the neuron further degrading it or leading to its death.
3) Alzheimer’s patients may experience issues with calcium regulation leading to a Ca2+ imbalance.
Calcium is very important and used in many ways by neurons including regulation of the activity of neurons. There are cell organelles called “calciosomes” that store and release calcium as needed. Calcium is also stored in the endoplasmic reticulum (which is a kind of assembly line that also has a structural function). There are proteins that bind with calcium in ways that help regulate it and transport it. Calcium is also stored within the mitochondria.
Diagram 7 of Mitochondria:

Mitochondria act as the cells “power plant”. Mitochondria take (fuel) food, protein, fat or glucose and convert it to the “electricity” of the cell which is a molecule called ATP that provides energy within the cell for all the various activities. The ATP changes form as it provides power from ATP to ADP and from there is typically recycled back to the mitochondria where it is “recharged” and changed back to the higher energy ATP.
Due to the fact that mitochondria provide most of the cells energy, they are also very active chemically. This chemical activity means they produce more free radicals and are themselves more exposed to the damaging effect of free radicals. Due to the fact that mitochondria are subject to more damage than other organelles, they are "repaired" or replaced fairly regularly. Replacing and repairing old and damaged mitochondria is essential for maintaining cellular health.
Given the size and length of axons and dendrites in neurons as well as the fact that the structure of neurons change with time as new memory and learning are created, mitochondria must both be regularly present and regularly move along the axon and dendrite to get to locations needing energy. Therefore, if the microtubules are damaged, or the cell is not operating correctly, it makes it difficult for the cell to manufacture new mitochondria to replace old ones. Old or damaged mitochondria are less efficient and produce more free radicals and ROS. This negatively impacts the health of the cells. In neurons, it is more difficult to replace mitochondria due to the fact that many are located in the periphery of the neuron – axon or dendrite. Thus, in neurons, mitochondria replacement is already slower. If something impairs the mitochondria replacement further, this degrades the ability of the neuron to function efficiently.
Cells that use a lot of energy such as certain muscle cells, organ cells such as the liver and neurons in the brain can have hundreds of mitochondria and mitochondria are an integral part of the cells “defenses” against virus and bacteria. There are various signaling mechanisms between the mitochondria, the cell nucleus and other organelles that are used to signal each other and regulate the activity of each. In fact, mitochondria are part of the "last ditch defense" of the cell. Under certain conditions, mitochondria trigger apoptosis or a type of programmed death of cells.
Viruses have co-evolved with animals and seek to take control of mitochondria activity in order to get the energy needed for viral replication and to prevent the cell from entering programmed death. If the signaling between mitochondria, the nucleus and other organelles is not working correctly, the mitochondria may not be replaced or repaired when they are damaged. Therefore, they may not provide sufficient energy. In some cases, mitochondrial damage should normally trigger cellular death but does not.
If calcium becomes deregulated, it can prevent proper functioning of the neuron and of the mitochondria. If the mitochondria are not working correctly, it is like having a power plant that is always experiencing brown outs or outages. The outages and brown outs can be very debilitating to the rest of the cell. Additionally, if the mitochondria are not operating efficiently, it produces more waste products than normal and worse waste products than normal (free radicals) and indirectly leads to the production of reactive oxygen species (ROS) which are toxic to the cell.
There are several other problems associated with calcium becoming deregulated. Basically, there are a cascade of “bad” events that can happened whenever normal calcium homeostasis is not maintained (homeostasis in this definition is the properly regulated and stable equilibrium of calcium that cells normally maintain).
4) The brain tissue of people afflicted with Alzheimer’s disease may become inflamed.
Our immune cells are designed to target foreign material, viruses, germs and our own malfunctioning cells. The immune system can change the way the cells operate such that they become “hardened” against certain types of infections or even radiation damage. Additionally, cells can secrete large numbers of chemicals (inflammatory factors) that signal the immune system that damage is occurring or has occurred. This spurs the immune system to take additional actions. Such action by the immune system can cause cell deaths. Some types of cellular death spur adjoining cells to secrete even more inflammatory factors. This in turn causes the immune system to become more involved in the area. If unchecked, inflammation can spur more inflammation and more damage. Other immune cells can secrete factors that reduce inflammation and bring the damage back under control. If not brought under control, then types of autoimmune diseases can occur. In Alzheimer’s patients, inflammation can increase the damage done to the brains of people suffering from this disease.
5) People with Alzheimer’s disease are often found to have malfunctioning mitochondria.
As mentioned earlier, the mitochondria act mainly as the cells power stations which provide usable energy in the form of ATP to the rest of the cell. In Alzheimer’s patients, mitochondria are often damaged or the neuron may contain too few mitochondria to match the energy expenditure needed by the cell.
Mitochondria are often depicted as round or kidney shaped organelles. Years ago, mitochondria were thought of as stable structures that live within the cell providing ATP when needed. But in modern time with better understanding of cellular activity, the understanding has changed. Mitochondria form a dynamic network within most cells where they constantly undergo fission and fusion. Mitochondria can divide in a type of binary fission where one mitochondrion divides into two. Each individual mitochondrion requires its own DNA to function. Therefore, a mitochondrion duplicates its DNA during fission. Two mitochondria can also fuse together forming a longer mitochondrion. Mitochondria are somewhat “mobile” within the cell and the position of the mitochondria within the cell can change. This is very important in the axon and dendrites of the neuron given their long length. A damaged mitochondrion that is fused together with another will have duplicate DNA. If there is damaged DNA in one of the mitochondrion, the duplication allows the combined mitochondrion to work better as one of the mitochondrion genomes is not damaged.
In neurons, fusion and fission of mitochondria is more important than in other cells. In fact, neurons are unable to survive without constant change of mitochondria through fusion and fission.
The cells maintain a type of homeostasis in mitochondrial fusion and fission to maintain the amount of mitochondria needed by the cell at the location they are needed. In a cell that has “decided” to die or is dying due to being “programmed” to die (called Apoptosis), the mitochondria break down into smaller organelles. The cells essentially destroy themselves in a way that is not very destructive to surrounding cells. Mitochondria also function in close association with the cells endoplasmic reticulum. Problems with mitochondria often have a large impact on the endoplasmic reticulum or vice versa. Part of the endoplasmic reticulum membrane is closely associated with the mitochondria. This is often called Mitochondria Associated endoplasmic reticulum Membrane (MAM).
Note: "ER" refers to endoplasmic reticulum and "Mito" to mitochondrion.
Diagram 8 Endoplasmic Reticulum and Calcium signaling:

Protein misfolding:
The endoplasmic reticulum is very important to folding, packaging and transport of most proteins in cells. Ribosomes are studded within the “rough” part of the endoplasmic reticulum. Ribosomes synthesize proteins and the endoplasmic finished the process by folding them, packaging them and even “labeling” them.
Diagram 9 of Cell nucleus and endoplasmic reticulum:

The endoplasmic reticulum is like a cellular "factory" that contains various things including ribosomes which are the machines in the factory that assemble parts together. This factory takes the instructions (given to it by RNA molecules that travel from the cell nucleus), and uses those instructions to create proteins, enzymes and other molecules and materials required by the cell or organism. When proteins are constructed, they may have molecules bound together sometimes in complex ways. The protein when complete is a 3D structure which can fold back in various ways. If it does not fold at the right places, the protein may have the right “formula” but not the right shape and will not work.
How does Anavex 2-73 (A2-73) help with Alzheimer’s disease?
The company writes that A2-73 is an orally available, small-molecule activator of the sigma-1 receptor restoring cellular homeostasis by targeting protein misfolding, oxidative stress, mitochondrial dysfunction, inflammation and cellular stress, factors in both neurodegenerative and neurodevelopmental diseases.
Diagram 10 Impaired Axonal Transport:

How does A2-73 restore cellular homeostasis? By acting as a sigma-1 agonist. Due to the complexity of cellular mechanisms, the control of certain aspects of cellular activity or metabolism is controlled by signaling molecules that bind to “receptors”. An agonist is a molecule or chemical that will bind to a “receptor” in such a way that the receptor is activated triggering a biological effect. The antagonist does the opposite – binds to the receptor and blocks the biological effect. By producing agonists and antagonists that act in various ways, cellular mechanisms are controlled. Note, receptors can themselves be complex. They can be activated and deactivated in different ways triggering different reactions by different agonists and antagonists.
Sigma-1 receptors are a type of membrane protein “chaperone” that move from the MAM to the membrane of other organelles depending on the stress and the presence and types of sigma-1 receptors or agonists. Sigma-1 receptors unlike some other receptors don't have an intrinsic activity where for example, if it is activated, it takes a specific action such as to release more or less of a neurotransmitter. Instead, the sigma-1 receptor "chaperone's" various types of reactions within the cell. Either inhibiting or promoting certain actions depending on the type of Sigma-1 ligand attached to the receptor.
As mentioned earlier, an agonist is a chemical that binds to a receptor and activates the receptor to produce a biological response. Whereas an agonist causes an action, an antagonist blocks the action of the agonist and an inverse agonist causes an action opposite to that of the agonist. The agonist and antagonist are both called “ligands”. A “chaperone” receptor such as the sigma-1 receptor is “mobile” in that it changes location due to cellular stress and the presence of ligands. In a typical cell, there might be various agonists and various antagonists for a particular receptor. There may be more than one type of agonist and more than one type of antagonist. When an agonist or antagonist binds to the receptor, it changes the 3 dimensional shape of the structure changing how it impacts various processes in the cell and even impacting where it is primarily located. Given the presence of various ligands that impact a particular receptor type, cellular interactions are complex.
Various receptors such as the sigma-1 receptor help regulate cellular processes. In particular, the sigma-1 receptor appears to have a large role in regulating “stress”. If activated in certain ways by the appropriate agonist, the sigma-1 receptor has been found to help regulate other cellular signaling molecules such as protein kinases and inositol phosphates and it helps regulate and modulate the activity of calcium channels. The regulation of those in turn impact many cellular stress related processes. Those processes influence inflammation, endoplasmic reticulum “stress” related issues such as “misfolded” protein formation and can impact the formation of Reactive Oxygen Species (ROS).
Latest Results for Alzheimer’s patients taking A2-73:
The average MMSE for people diagnosed with Alzheimers is around 24 to 26. These patients that are not treated on average have a MMSE decline of 4.3 over the first 2 years. In the next 2 years (4 years total), their MMSE would decline another 7.66 points. They would be down a total of 11.96 points.
The data from patients taking high concentrations of A2-73 for 146 weeks show a MMSE decline of about ~ 1.6. If the trend continues, by 6 years, there would be a MMSE point decline of 3.3 and by 9 years, there would be a MMSE decline of 4.8.
The patients treated for almost 3 years with high concentrations of A2-73 have a drop of ~1.6. The decline in MMSE patients treated with high concentration of A2-73 is less than1/3 of the untreated patients.
(Based on an Anavex’s original phase 2A test of which there are 8 people who are taking high concentrations of A2-73 and there are 13 people taking low to medium concentrations of A2-73. There is a very large difference between the people taking high concentrations of A2-73 and those taking low concentrations of A2-73).
Diagram 11 MMSE results high concentration versus low/medium concentrations of A2-73 (diagram from Anavex presentation):

What does this mean? It means that the average person who gets Alzheimer's after age 70 who takes a high dose of AVXL 2-73 would be able to live out their life until death with a much lower reduction in ability to think and remember. This would result in patients still remembering their life while being able to live on their own if they are otherwise healthy enough to do so.
Additional posts from Amstocks, most recent to earlier:
amstocks82 Tuesday, 02/18/20 02:26:12 PM
Re: None 0
Post #
237029
of 271800
Alzheimer's disease in the USA and the future of Anavex
An estimated 5.8 million Americans of all ages are living with Alzheimer’s dementia in 2019. This number includes an estimated 5.6 million people age 65 and older and approximately 200,000 individuals under age 65 who have younger-onset Alzheimer’s, though there is greater uncertainty about the younger-onset estimate.
Of the 5.8 million people who have Alzheimer’s dementia, 81 percent are age 75 or older (Figure 1).
Out of the total U.S. population:
• One in 10 people (10 percent) age 65 and older has Alzheimer’s dementia
• The percentage of people with Alzheimer’s dementia increases with age: 3 percent of people age 65-74, 17 percent of people age 75-84, and 32 percent of people age 85 and older have Alzheimer’s dementia.
Source: https://www.alz.org/media/Documents/alzheimers-facts-and-figures-2019-r.pdf
The number of people with Alzheimer's disease in the world is estimated to be ~ 44 million.
There are over 2 million new Alzheimer's patients per year.
Assume that around 3 million people in the world would have the resources to treat Alzheimer's disease and would buy A2-73. That is a huge population. Given the patent protection, and relative low cost of making A2-73, Anavex could easily get $1,000 per patient per year (after shipping, handling and manufacturing costs). That is $3 billion new free cash flow per year just for Alzheimer's disease.
Some of the new drugs in the pipeline from other companies that could work for Alzheimer's disease are IMO likely to be drugs that could be taken together with A2-73. The ones I've looked at from other companies have other different mechanisms of action not related to the mechanism of A2-73. There are also other diseases like Parkinson disease that would add to the number of potential patients for A2-73. My view is that since the mechanism of A2-73 is to reduce cellular stress and cellular inflammation, A2-73 would likely end up being helpful for other autoimmune related diseases. This is of course only speculation at this time. But it would not be the first time drugs that are developed for one thing end up being used for many. What all of it adds up to is that A2-73 and their follow on drugs would have a very wide range of application and they could go from an unknown small company to a large company in literally a year or two.
amstocks82 Thursday, 02/13/20 02:11:20 PM
Re: None 0
Post #
236168
of 271800
The Advantage of Fast Track status for Anavex 2-73
Most new drugs have huge manufacturing costs. For example, many of the new drugs are classified as biologics. A factory to make a biologic drug cost around $200 to $400 million. Biologics are complex molecules which typically are mainly proteins. Quite often, they are made inside of living cells that are grown in the manufacturing plant. The cells are often coaxed to make the protein via genetic engineering. I.e:
http://theconversation.com/biologics-the-pricey-drugs-transforming-medicine-80258
Anavex has a small molecule drug that can be much more easily manufactured. The company can hire many different labs to manufacture their drug for a very reasonable price. This is in contrast to some biologic drugs which may cost $40,000 to $50,000 per patient just to manufacture the drugs that they need not including the cost of R&D and profit.
My rough estimate is that Anavex could manufacture the drugs a patient needs per year for ~ $1000.00 per patient in a quality facility or they can build their own facility when they are ready to sell in volume.
If Anavex is able to get the drug approved for any indication via a fast track process, - for example for RETT syndrome, then once it is approved, other doctors will consider trying it for different diseases so long as the disease has no satisfactory medicine. Typically, insurance companies won't pay for the off-label use. But doctors will prescribe. This actually happened to one of my family members. A doctor prescribed the medicine for 2 years before it was finally approved. Meaning out family paid the full price for 2 years. This was obviously expensive costing over $6,000 dollars a month. But nothing else worked.
If Anavex 2-73 is approved for RETT syndrome, then the drug becomes commercially available. Doctors can look at the clinical trial results and decide to ask their patients if they would like to try Anavex 2-73 for Parkinson's disease or Alzheimer's disease. Here is a reference:
https://www.webmd.com/a-to-z-guides/features/off-label-drug-use-what-you-need-to-know#1
Given the low cost of manufacturing Anavex 2-73, the Anavex can sell the drug for a low enough price that it can be essentially sold to a mass market. If Anavex 2-73 were to be sold to 1,000,000 patients and the company sells the drug such that they make $1,000 (after manufacturing and distribution costs), that would amount to $1 billion in free cash flow.
Lots of excitement here .....
amstocks82 Wednesday, 02/12/20 01:22:42 PM
Re: None 0
Post #
235804
of 271800
Latest Presentation....
The presentation: https://www.anavex.com/wp-content/uploads/2020/02/Anavex-Presentation-February-2020.pdf
contains some new information that I think is very significant.
Slide 8: Left half of slide shows is how well the drug activates the sigmar-1 receptor. The drug is supposed to work by activating the Sigmar-1 receptor. The data is based on brain imaging PET scans. These show that indeed the drug does occupy the receptors and that occupancy is related to the dose.
So, the next question would be is whether occupancy of the sigmar-1 receptor will really have a positive impact on the illnesses being tested for?
On the right side of slide 8, this shows corresponding changes in functional outcomes based on dose. This shows a strong correlation between the theory that sigmar-1 receptor occupancy should help with the disease.
Going forward in the presentation...
From slide 14, here is some of the p values....
REPORT on PART A: INTENSIVE PK SUBCOHORT
• Plasma levels of the biomarker Glutamate decreased significantly (Week 0 vs. Week 7; 2-tailed
Wilcoxon signed rank test, p = 0.046)
• Levels of Glutamate at Week 7 directly correlated with CGI-I scores at Week 7 (2-tailed
Spearman’s rho = 0.837, p = 0.038)
• Greater decreases in Glutamate associated with greater improvement in these efficacy scores
• GABA changes demonstrated an inverse correlation of the magnitude of Glutamate changes (2-
tailed Spearman’s rho = -0.829, p = 0.042)
===================
The p values of 0.05 or less is considered significant meaning the result is likely significant. All the p values show it is likely significant. More over, since there are more than one test, it means that the results can be considered to be more significant.
For slide 19, Anavex was wise to continue to collect data. The data shows that there is a very significant difference between low to medium dose patients and high dose patients. The number of patients is small - but given they are at 148 weeks, the results are very significant with very low p values. Since a p value of less than 0.05 is considered to be significant, the p value of p < 0.0001 is the best I've noticed in any presentation on a new medicine.
How significant is it? Consider a p < 0.05. If a person thought that A 2-73 didn't work and calculated a p value for it and it was p < 0.05, then it means that
A p < 0.05 means there is a 5% chance that the observed drug results were due to just random error. If p < 0.01, then it would be a 1% ... When you get to p < 0.0001, then the probability is 0.001 %. Not very large.
The more time there is and the result remains consistent, the more likely it is that the results seen are not some random statistical anomaly. Of course, there might be some other unknown variable impacting the result introducing some error - but I can't think of one especially given the MMSE results are correlated with the ADSL over that time range.
Everything looks good so far....
Recent AVXL News
- Contact The Gross Law Firm by May 13, 2024 Deadline to Join Class Action Against Anavex Life Sciences Corporation(AVXL) • PR Newswire (US) • 04/19/2024 09:45:00 AM
- The Gross Law Firm Reminds Shareholders of a Lead Plaintiff Deadline of May 13, 2024 in Anavex Life Sciences Lawsuit - AVXL • PR Newswire (US) • 04/16/2024 09:45:00 AM
- Class Action Filed Against Anavex Life Sciences Corporation (AVXL) - May 13, 2024 Deadline to Join - Contact The Gross Law Firm • PR Newswire (US) • 04/12/2024 09:45:00 AM
- Anavex Life Sciences to Present at the Noble Capital Markets Virtual Healthcare Equity Conference • GlobeNewswire Inc. • 04/11/2024 11:30:00 AM
- Class Action Filed Against Anavex Life Sciences Corporation (AVXL) - May 13, 2024 Deadline to Join - Contact The Gross Law Firm • PR Newswire (US) • 04/09/2024 09:45:00 AM
- Anavex Life Sciences Corporation Class Action: The Gross Law Firm Reminds Anavex Life Sciences Investors of the Pending Class Action Lawsuit with a Lead Plaintiff Deadline of May 13, 2024 - AVXL • PR Newswire (US) • 04/05/2024 09:45:00 AM
- Shareholders that lost money on Anavex Life Sciences Corporation(AVXL) should contact The Gross Law Firm about pending Class Action - AVXL • PR Newswire (US) • 04/02/2024 09:45:00 AM
- Lost Money on Anavex Life Sciences Corporation(AVXL)? Join Class Action Suit Seeking Recovery - Contact The Gross Law Firm • PR Newswire (US) • 03/29/2024 09:45:00 AM
- Investors who lost money on Anavex Life Sciences Corporation(AVXL) should contact The Gross Law Firm about pending Class Action - AVXL • PR Newswire (US) • 03/26/2024 09:45:00 AM
- Anavex Life Sciences to Present at the 23rd Annual Needham Virtual Healthcare Conference • GlobeNewswire Inc. • 03/25/2024 11:30:00 AM
- The Gross Law Firm Notifies Anavex Life Sciences Corporation Investors of a Class Action Lawsuit and Upcoming Deadline • PR Newswire (US) • 03/22/2024 09:45:00 AM
- Anavex Life Sciences Initiates Placebo-Controlled U.S. Phase 2 Clinical Trial of ANAVEX®3-71 in Schizophrenia • GlobeNewswire Inc. • 03/18/2024 11:30:00 AM
- Anavex Life Sciences to Present at the 44th Annual TD Cowen Health Care Conference • GlobeNewswire Inc. • 02/26/2024 12:30:00 PM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 02/23/2024 11:05:18 AM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 02/23/2024 11:04:32 AM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 02/23/2024 11:04:31 AM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 02/23/2024 11:03:48 AM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 02/23/2024 11:03:06 AM
- Form 10-Q - Quarterly report [Sections 13 or 15(d)] • Edgar (US Regulatory) • 02/07/2024 09:31:07 PM
- Anavex Life Sciences Reports Fiscal 2024 First Quarter Financial Results and Provides Business Update • GlobeNewswire Inc. • 02/07/2024 12:30:00 PM
- Anavex Life Sciences to Announce Fiscal 2024 First Quarter Financial Results on Wednesday, February 7th, 2024 • GlobeNewswire Inc. • 02/01/2024 12:30:00 PM
- Form SC 13G/A - Statement of acquisition of beneficial ownership by individuals: [Amend] • Edgar (US Regulatory) • 01/25/2024 10:01:34 PM
- Anavex Life Sciences Reports Publication of ANAVEX®3-71 in Clinical Journal Confirming Pharmacokinetic Dose Proportionality of ANAVEX®3-71 in Humans • GlobeNewswire Inc. • 01/24/2024 12:30:00 PM
- Anavex Life Sciences Announces U.S. Phase 2 Clinical Trial of ANAVEX®3-71 in Schizophrenia • GlobeNewswire Inc. • 01/16/2024 12:30:00 PM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 01/13/2024 01:00:34 AM
Axis Technologies Group and Carbonis Forge Ahead with New Digital Carbon Credit Technology • AXTG • Apr 24, 2024 3:00 AM
North Bay Resources Announces Successful Equipment Test at Bishop Gold Mill, Inyo County, California • NBRI • Apr 23, 2024 9:41 AM
Epazz, Inc.: CryObo, Inc. solar Bitcoin operations will issue tokens • EPAZ • Apr 23, 2024 9:20 AM
Avant Technologies Launches Advanced AI Supercomputing Network and Expansive Data Solutions • AVAI • Apr 23, 2024 8:00 AM
BestGrowthStocks.com Issues Comprehensive Analysis of Triller Merger with AGBA Group Holding Limited • AGBA • Apr 22, 2024 1:00 PM
Cannabix Technologies to Present Marijuana Breathalyzer Technology at International Association for Chemical Testing (IACT) Conference in California • BLO • Apr 22, 2024 8:49 AM











