Register for free to join our community of investors and share your ideas. You will also get access to streaming quotes, interactive charts, trades, portfolio, live options flow and more tools.
NWCI is severely delinquent in filing the company's Financials. The stock is at risk of an SEC Suspension and subsequent stock registration revocation.
https://www.sec.gov/cgi-bin/browse-edgar?company=Newcardio&owner=exclude&action=getcompany
What a low life pos this dude is. How do these guys sleep at night
Really does suck, these guys real p o s how they sleep at night, I don't know
NWCI been abandoned, those who set us up to buy, with those PMs and Txts, have cashed out and gone.
I can name 6 stocks that the same frontloader group, unloaded on there alerts.
Enough to wreck a whole lot of good money, following that PSS Psycho
All we can do is hope
WATCH BOARD ON MONDAY NWCI SIGNS OF LIFE
SETUP 4 MONDAY COULDN'T B BETTER. GOING UP!
WOW CAME BACK AS A BULL AT CLOSE; MONDAY=GOING UP!!
Nite has picked up every share on the bid imo looks like nice accumulation going on
I'm in...don't let me down
I don't think Nite wants too many slaps on the ask ;)
fill a few bids accumulation . could see a penny again IMHO
Looking good for move up$$:)
LMAO, yes this is a clean trading shell and dark reporting so SS can not be changed. RM play sends it to .10 easy IMO.
Opportunities present themselves in a variety of ways in the OTC$$$:)I prefer to focus on the trading aspect$$$:)gltu
The voice of reason...refreshing!
HPT
Do any of you folks who keep posting on this site even know what you're talking about?
NWCI was basically taken over in January of 2012 by two companies that had been major investors and in April the assets of this company were auctioned off. Everything that you see on this website above these posts no longer exists.
There is no company, There are no medical patents as assets. There is nothing but a shell of a company that could be used to back in another company. That's it.
Before any of that could happen however, the folks who currently control this shell - and there's no assurance that that company is still Vision Capital - would have to clean up the non-existent paperwork that would be required for this to happen.
As of this morning the last filing by NWCI to the SEC took place on January 10, 2012. So all of this crap about it's going to bounce, it's going to take off, it's going to make you a millionaire is all bullshit. There's nothing to support a bounce except bullshit and pipe dreams.
It's true that this shell could be used to jump start a new company, but unless and until that process starts taking place with the SEC, then this is much ado about nothing.
Oh yeah, if you bother to read the original corporate documents for this company, you'll see that the board of directors have the right to reduce your shares by as much as 95% if a new company were ever to be backed in to this shell.
Good luck.
Nice bottom accumulation$$$:)NWCI primed for a BIG bounce$$$:)
Hey Rainer someone deff accumulating added few myself
its up from the initial dip this morning. Watching closely
Somebody keeps buying up shares cheap here!
We could still see a few bounces here
looks like its over
Let's see a springboard type bounce here
NWCI bouncer when the stop sign comes off?
Nice moves it bounced around 50% the last time it dipped like this
As anticipated 1/3 value washed away this morning. Hope some of the unsuspecting folks were able to get out prior to recent lows.
Some real stupidity written on this board. Warned u guys last week!
So am I man. Sounds like it
could be a big week for all
of us who bought in! @D
GO NWCI!
$$$$$$$$$$$$$$$
mikewazowski
NWCI
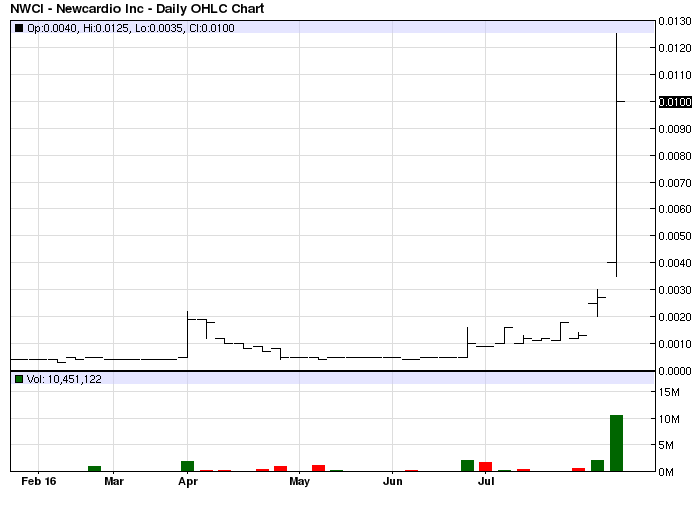
Sad chart, but does show actual value of shares being pumped
He is great guy try's to help many
Yes, great opportunity to dump shares on unsuspecting traders. Sad trading with this OTC PS.
Great seeing you here BA. I've been on a few different boards and stocks with you. You're good people and when something gets your attention, it's often a good opportunity. Have a great weekend.
NWCI nice close bottom is in next week fun begins imo :)
$NWCI
|
Followers
|
29
|
Posters
|
|
|
Posts (Today)
|
0
|
Posts (Total)
|
568
|
|
Created
|
10/09/08
|
Type
|
Free
|
| Moderators | |||
|
|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||
 OTC Pink No Information OTC Pink No Information | Corporate Headquarters | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
|  | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
 | NewCardio, Inc. (OTC BB: NWCI), a cardiac diagnostic and services company, develops proprietary platform technology to dramatically improve accuracy and significantly increase the diagnostic value of the standard 12-lead electrocardiogram (ECG). NewCardio's solutions incorporate novel, state-of-the-art technology that management believes will significantly improve the diagnostic accuracy and precision of the analysis and use of signals from ECGs, without disrupting the well-established 12-lead ECG practice. The Company's technology is based on a three-dimensional approach to modeling cardiac activity using standard 12-lead ECG signals. Accordingly, New-Cardio is poised to, over time, derive value from each of the 375 million ECGs performed annually around the world. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| NewCardio is applying its proprietary technology to assess cardiac safety of new drugs in development and to improve clinical diagnosis and assessment of patients with suspected and established heart disease. Management believes this technology represents a highly significant advance and has the opportunity to be a disruptive force in markets that exceed $4.5 billion in aggregate annually. |  | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
| Management includes thought leaders from the Intel, pharmaceuticals, medical devices (St. Jude Medical), Core Labs and the investment industry. The Company believes its broad-based management team is particularly wellsuited to advance the Company's goals and develop its technology into a set of essential diagnostic tools for the ECG segment of the cardiac diagnostics industry. Key initial applications include cardiac toxicity (lethal arrhythmias) determination during new drug development,and cardiac status for acute and chronic patient management. The FDA requires that all new drugs be tested for potential cardiac toxicity early in development. Current methods are costly and timeconsuming. New drugs are often tested on more than 1,000 subjects, with ECGs collected at multiple time points. Using NewCardio's 3D approach, drug sponsors and CROs can automate this process, significantly reducing the cost and accelerating the test times for drugs in development. A leading independent industry analyst estimated this market to exceed $650 million annually. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| NewCardio's products could change the way patients with chronic heart conditions will monitor their disease outside the acute care setting. The Company is developing a hand-held device that could transmit the ECG data and synthesized 12-lead data to doctors remotely. This will enable complete cardiac assessment, in contrast to current devices that enable arrhythmia monitoring only. A recent study by Frost & Sullivan estimated this market to approach $2 billion. The Company believes its diagnostic tools will substantially improve the accuracy and timeliness of the diagnosis and response to patients arriving at Emergency Departments with suspected acute coronary syndromes (heart attack and related conditions). NewCardio's My3KG will enable doctors to diagnose acute coronary syndromes more quickly and accurately than with traditional methods, possibly saving thousands of lives annually and reducing costs associated with unnecessary hospital admissions. With more than 70 million ECGs performed in ERs each year in the U.S. alone at an average (current) reimbursement of $30 each, this market could exceed $2 billion annually at current reimbursement rates. | NewCardio was selected from MDB's 2011 group of "Best and Brightest" small-cap companies, a group that is advancing some of today's most innovative and market-leading intellectual property (IP). It is one of the 40 public companies ranking in the 90th percentile for its respective technology leadership from more than 1,500 small-cap companies with granted U.S. patents, as rated by PatentVest, MDB's proprietary IP business intelligence platform. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| NewCardio Receives Notice of Allowance for Vital Patent for Cardio3KG(TM) Represents Core Patent for NewCardio's 3-D ECG platform technology SANTA CLARA, Calif., Feb. 25 /PRNewswire-FirstCall/ -- NewCardio, Inc. (OTC Bulletin Board: NWCI) a cardiac diagnostic technology provider, announced today that it has received a Notice of Allowance from the United States Patent and Trademark Office (USPTO) for a patent covering technology relating to NewCardio's Cardio3KG solution (formerly VisualECG or Visual3Dx). This represents the core patent for NewCardio's anticipated emergency/urgent care solution, Cardio3KG. The Cardio3KG solution is comprised of a set of algorithms and tools that provide a comprehensive method to assess cardiac electrical activity in time and space, and to extract additional 3-dimensional information from standard 12-lead ECG signals. This may enable Cardio3KG to assess potentially fatal diseases and conditions, including acute coronary syndrome, with greater speed and accuracy than possible by standard ECG. When issued, together with US patent 7,266,408,this will be NewCardio's second U.S. patent covering broad aspects of the Company's 3-D ECG platform technology and, particularly, specifics of Cardio3KG, the first solution targeted to take full advantage of this intellectual property. "This action by the USPTO reinforces our continuing investment in and commitment to our novel cardiovascular diagnostic platform technology, which provides 3-D analysis of the heart's electrical activity," stated NewCardio's CEO, Branislav Vajdic, PhD. "We believe the Cardio3KG solution will substantially improve the accuracy and timeliness of response and diagnosis for patients arriving at emergency departments with heart attack symptoms and related conditions. NewCardio's Cardio3KG will enable doctors to diagnose acute coronary syndromes more quickly and accurately than with traditional methods, possibly saving thousands of lives annually and reducing costs associated with unnecessary hospital admissions. We also believe Cardio3KG, along with QTinno™ and CardioBip™, represent important steps forward in validating our 3-D ECG platform's ability to improve cardiac diagnosis, clinical outcomes, and drug safety. We are pleased that patent offices in both the US and Europe are acknowledging our proprietary claims to our core technologies." | PATENT PROTECTED
|
| App Number/ Filing Date
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Newcardio Inc, has been invited to present the results of a recent QTinno ® performance study at an upcoming conference entitled "Cardiovascular Safety in Drug Development: State-of-the-Art Assessments," sponsored by the U.S. Food and Drug Administration (FDA), the Cardiac Safety Research Consortium (CSRC) and the Heart Rhythm Society (HRS). The conference will be held in Washington, DC, on April 14-15, 2011. Dr. Ihor Gussak, NewCardio's Chief Medical Officer, commented, "We were delighted with the significant improvement in measurement variability and study power that we observed in this investigation. We look forward to sharing this exciting data at this important conference, which is attended by cardiovascular safety experts from regulatory bodies, pharmaceutical companies, clinical research organizations and academia. The improvement conferred by our new automated ECG selection algorithm offers sponsors the opportunity to reduce the number of subjects in a study without sacrificing study power, which may result in substantial cost savings. The new algorithm is a powerful extension of our platform technology, and further demonstrates NewCardio's ability to add value to every ECG." Vincent Renz, CEO of NewCardio, commented, "These study results represent yet another important and substantial technological innovation for QTinno that further differentiates QTinno from competition and solidifies QTinno's position as the industry's leading automated solution for drug safety studies. This is further evidence that NewCardio will continue to enhance QTinno in order to eliminate the manual, labor intensive procedures employed in the current cardiac safety methodologies, which typically affect the drug sponsors through longer timelines, higher costs and from our findings, lower quality. We believe that our latest innovation, intelligent ECG extraction, will provide additional gains that will allow the pharmaceutical industry to complete higher quality cardiac safety analysis faster, more reliably, and at significantly lower cost." | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
NewCardio Study Shows my3KG Improves Diagnostic Accuracy in Diabetics with Acute Myocardial Infarction. The presentation will be made at the 71st Annual ADA Scientific Sessions to be held in San Diego, CA, June 24-28, 2011. In addition, the ADA has selected this presentation to be showcased in the Guided Audio Poster Tour, which features expert moderators that share their perspectives with attendees, highlighting novel and important developments in the field. Dr. Ihor Gussak, MD, PhD, NewCardio's Chief Medical Officer, commented, "We are delighted to present our study results at this important gathering of diabetes experts, and particularly pleased that the ADA program committee chose to feature our presentation in a moderated poster session. Accurate and timely AMI diagnosis is a matter of great concern for diabetologists, and we believe our results show that my3KG can play a major role in solving it. The moderated poster session provides the opportunity to present our results directly to leading diabetes experts, and have one-on-one interactions that will allow us to discuss and explain the importance of our results in detail." | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Leadership and AdvisorsThe Company has assembled a team of talented and experienced entrepreneurs and business managers, including leaders from various industries like technology, pharma, academics, and a wide range of other scientists and professionals.
Management TeamNewCardio has assembled an experienced and skilled management team which has proven their ability to create new products and build new enterprises. Jess Jones, M.D. - Chief Executive OfficerDr. Jones has been a member of the Company's Board of Directors since December 2008 . From 2006 to December 2010 Dr. Jones worked with Vision Capital Advisors, LLC in New York City as the Director of Healthcare Investing, analyzing investment opportunities in the biotechnology, pharmaceutical, medical technology, and medical services fields, and assisted companies in implementing their business plans. From 2001 to 2007, Dr. Jones attended Columbia College of Physicians & Surgeons in New York City , where he received his medical degree in May 2007 . Additionally, Dr. Jones earned a BA degree from the University of Utah in 2001 and an MBA from Columbia Business School in May 2007. Greg Sadowski - Chief Operating OfficerMr. Sadowski has served as Senior Vice President, Client Services for NewCardio since October 2008 . In that position, he has been responsible for preparing, delivering and supporting the NewCardio cardiovascular diagnostic technology solutions, including the Professional Services, Information Technology, Customer Care and Quality Assurance groups. From October 1997 to October 2008 , Mr. Sadowski worked with eResearchTechnology (eRT), where he served on the Executive Management Team as Senior VP of eRT's Electronic Patient Reported Outcomes (ePRO) business. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Board of Directors Michael E. Hanson Mr. Hanson is a founding partner of Barnard Life Sciences, a healthcare consulting company founded in 2001. From 2004 to 2009, Mr. Hanson was a member of the board of directors, compensation and audit committees, of Indevus Pharmaceuticals. From 2002 to 2006, Mr. Hanson was a member of the board of directors, compensation and audit committees, of GlycoGenesis, an oncology-focused company. From 1998 to 2001, he was a member of the board of directors, compensation and audit committees, of MGI Pharma, Inc., which was later purchased by Eisai Pharmaceuticals. Mr. Hanson also serves on the board of directors of Z-92 Pharma and Eleos, Inc., and is also a member of Pearl Street Ventures, a venture capital firm that specializes in healthcare companies and Cardinal Equity Partners, a private equity firm. Previously, Mr. Hanson spent 25 years in positions of increasing responsibility at Eli Lilly and Company. He served as President and General Manager of Eli Lilly Japan KK from 1989 to 1992. He subsequently served as Vice President of Lilly Research Laboratories with responsibilities for the Medical Department. He culminated his Lilly career as President of the Internal Medicine Business Unit which included all cardiovascular and oncology products and became a member of the Eli Lilly and Co. Operations Committee (the senior management group of the company at that time). Mr. Hanson received a B.S. in Pharmacy from North Dakota State University and an M.S. in Hospital Pharmacy Administration from the University of Minnesota, and attended the Advanced Management Program at Harvard Business School. Jess Jones, MDDirector since December 1, 2008. From 2006 to present Dr. Jones has worked with Vision Capital Advisors, LLC as the Director of Healthcare Investing, analyzing investment opportunities in the biotech, pharmaceutical, medical technology, and medical services fields, and assisted companies in the implementation of their business plans. From 2001 to 2007, Dr. Jones attended Columbia College of Physicians & Surgeons, where he received his medical degree in May 2007. In 2005, while attending Columbia Medical School, Dr. Jones was awarded an American Heart Association-Medical Student Research Fellowship to study post-stroke inflammatory mediators in the Department of Neurosurgery. Additionally, Dr. Jones earned a BA degree from the University of Utah and an MBA from Columbia Business School. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Professional AdvisorsMorrison & Foerster LLPWorldwide IP Attorney Wilson Sonsini Goodrich & Rosati LLCCorporate Attorney Russell Bedford Stefanou Mirchandani LLPAuditors |
| Name of Beneficial Owner | Common Stock Beneficially Owned (1) | Percentage of Common Stock (2) | ||||||
| Branislav Vajdic, Ph.D. (3) | 9,208,910 | 27.88 | ||||||
| Vincent W. Renz, Jr. (4) | 1,237,137 | 3.89 | ||||||
| Richard Brounstein (5) | 690,000 | 2.19 | ||||||
| Mark W. Kroll, Ph.D., FACC, FHRS (6) | 419,896 | 1.34 | ||||||
| Robert N. Blair, M.Inst.P. (7) | 830,843 | 2.67 | ||||||
| James A. Heisch (8) | 228,790 | * | ||||||
| Jess Jones, M.D. | 0 | * | ||||||
| Patrick Maguire, M.D., Ph.D. (9) | 177,956 | * | ||||||
| Michael Hanson (10) | 145,877 | * | ||||||
| Ihor Gussak (11) | 786,705 | 2.49 | ||||||
| Dorin Panescu (12) | 755,777 | 2.39 | ||||||
| Greg Sadowski (13) | 429,208 | 1.38 | ||||||
| All officers and directors as a group (12 persons) | 14,911,099 | 39.48 | ||||||
Please keep this board about NWCI only!
| Volume | |
| Day Range: | |
| Bid Price | |
| Ask Price | |
| Last Trade Time: |
