Register for free to join our community of investors and share your ideas. You will also get access to streaming quotes, interactive charts, trades, portfolio, live options flow and more tools.
No doubt, buying in ASAP
Great time to get in now, 800% growth in six months. Demand for generics is high, and their otc products have great margins!
VANC Pharmaceuticals Announces Appointment of Mr. Bob Rai to its Board of Directors
VANCOUVER, BRITISH COLUMBIA--(Marketwired - June 5, 2015) - VANC Pharmaceuticals Inc. (TSX VENTURE:NPH)(OTCQB:NUVPF) ("VANC" or the "Company"), a pharmaceutical company focused on the Canadian generic drug and over-the-counter (the "OTC") markets, is pleased to announce that Mr.Bob Rai has been appointed to the Company's Board of Directors effective immediately. Mr. Rai is a pharmacist with over 15 years of experience and is partner in a chain of The Medicine Shoppe Pharmacy (www.pharmacybc.com).
"We are excited to have someone with Mr. Rai's industry experience, track record as an entrepreneur and global pedigree join our board. We welcome him to our growing team and eagerly await his input on the strategic direction of our Company," said Arun Nayyar, CEO of VANC. "As we begin the commercialization of our product portfolio it is crucial that our team be optimized and Mr. Rai's direct experience in the Canadian retail and institutional pharmacy business is going to be an immediate value add and impactful addition for the Company."
Mr. Rai is a graduate of the University of British Columbia with Bachelor of Science Degrees in Biochemistry and Pharmaceutical Science. In addition to operating a chain of The Medicine Shoppe Pharmacies in Greater Vancouver for the past 15 years, he has had several successful entrepreneurial and charitable endeavors. In 1998 Mr. Rai and his partners pioneered and revolutionized the online pharmacy business to the United States. The online sales and distribution of prescription medicines saw unprecedented industry growth and as other operators followed suit, the unique business concept became a billion dollar industry across Canada.
Mr. Rai is also Chairman and CEO of Canada Pacific Global Pharmaceuticals and Chairman of its subsidiary, PharmaCanada Inc. (www.earlycancerdetect.com). He has served as President of the Philippines Canada Trade Council (PCTC) from 2006-2007 and held the position of Vice-President from 2004-2006. As President of PCTC, he led a successful Trade Mission to Manila with endorsements from His Excellency Canadian Prime Minister Stephen Harper, Honorable Premier Gordon Campbell of British Columbia and Minister of International Trade and Industry of Canada David Emerson.
In 2013 Mr. Rai was the recipient of the Queen's Diamond Jubilee Medal for his over 18 years of community and volunteer work. His charitable and community volunteer activities include: Alumni UBC Advisory Council representing the Faculty of Pharmaceutical Science, Director of Tapestry Foundation for Health Care, Rotary Club of Vancouver Fraserview and cabinet member of "A Night of Miracles" for BC Children's Hospital.
"I am impressed with how far VANC has come as a startup in a highly competitive industry and commend the existing team for building a product portfolio, registering it with Health Canada and rolling it out commercially in such a cost and time efficient manner. I look forward to working with the team and helping them with governance, technology, product acquisition and accelerating sales," said Mr. Bob Rai.
The Company has also set aside 400,000 incentive stock options at $0.45 for a period of five years and 800,000 incentive stock options at $0.55 for a period of two years to Directors, Officers and Consultants.
On behalf of:
VANC Pharmaceuticals Inc.
Arun Nayar, President & CEO
Cautionary Note Regarding Forward-looking Statements: Information in this press release that involves VANC's expectations, plans, intentions or strategies regarding the future are forward-looking statements that are not facts and involve a number of risks and uncertainties. VANC generally uses words such as "outlook," "will," "could," "would," "might," "remains," "to be," "plans," "believes," "may," "expects," "intends," "anticipates," "estimate," "future," "plan," "positioned," "potential," "project," "remain," "scheduled," "set to," "subject to," "upcoming," and similar expressions to help identify forward-looking statements. The forward-looking statements in this release are based upon information available to VANC as of the date of this release, and VANC assumes no obligation to update any such forward-looking statements. Forward-looking statements believed to be true when made may ultimately prove to be incorrect. These statements are not guarantees of the future performance of VANC and are subject to risks, uncertainties and other factors, some of which are beyond its control and may cause actual results to differ materially from current expectations.
Neither the TSX Venture Exchange nor its Regulation Services Provider (as that term is defined in the policies of the TSX Venture Exchange) accepts responsibility for the adequacy or accuracy of this release.
VANC Pharmaceuticals Inc.
Arun Nayar
President & CEO
anayar@vancpharm.com
Investor Relations:
Kam Thindal
+ 1 (604) 566-9233
kam@htcapitalcorp.com
Read more: http://www.digitaljournal.com/pr/2575920#ixzz3cCNuUdVv
New Article on $NPH
"Vanc Pharma's Road to Commercialization"
From the team at TradersBoardroom.com
Read it here:
http://tradersboardroom.com/2015/05/13/nph-vanc-pharmas-road-to-commercialization/
Whats with the French news? What was it halted for?
It's time to rock.
"This stocks on fire" could be a new song...
Company has gotten noticed - just wait until the end of Q4 2015 - we could see impressive sales figures ??
2 days ago CXR bought Covis for $1.2 Billion. Covis had 18 generic branded products
''The Covis drug portfolio being acquired consists of 18 branded and authorized generic products with stable revenue, strong margins and free cash flow. The distinctive product portfolio includes branded pharmaceuticals, injectables and authorized generics that address life threatening and other serious medical conditions in various therapeutic areas including cardiovascular, central nervous system, oncology and acute care markets.''
Source
NPH has 49 products..(with 45 million shares out you do the math...)
CURRENT SATUS
Cross-referencing agreement – 30 products from globally reputed generic companies
Drug establishment license • Received in December 2014
Regulatory approval from Health Canada
• Submitted ANDS application for 33 products and in process of submission for remaining products
• Received 67 DINs
• Remaining 16 Products to be filed in Q1-2015 with Health Canada
SOURCE
Vanc Pharmaceuticals Inc. has signed a letter of intent with a multichain pharmacy based in Western Canada to become a preferred supplier of generic drugs and over-the-counter products.
SOURCE
Vanc Pharmaceuticals Inc. has received confirmation from the B.C. Ministry of Health approving 14 of the company's generic molecules under the Low Cost Alternative program.
The approval of these molecules makes them eligible for listing on British Columbia's provincial formulary and for reimbursement through Pharmacare. Pharmacare is the province's coverage plan that assists residents with the cost of eligible prescription drugs and certain medical supplies.
"We are excited to have received LOA approval from the B.C. Ministry of Health as it signifies an important milestone in our evolution as a company -- we can now provide residents of B.C. with safety, quality and affordability when it comes to these 14 molecules," said Arun Nayyar, chief executive officer of Vanc. "We will continue to work with the Ministry of Health to gain approval of additional molecules during Q1 2015."
source
Next Steps
Ordering – Purchase orders placed for 30 products
Packaging and Artwork – Ready for 30 products and in process of gaining approvals from manufacturers Manufacturing
– Q1-2015 Provincial Listing
– Applied for all products Sales and Marketing
– Expand sales and marketing team Q2-2015
QC – testing method transfers are in progress and agreement in place with an Ontario based testing lab
Yes if makingmoneynow (of Stockhouse fame) knows of what he speaks, this stock could be a serious money maker. Could make up my deficit and then some. Just not sure I'm ready to add yet; although I'm contemplating it.
THIS STOCK IS ON FIRE
Get ready folks, this is setting up to be the biggest play of 2015
Great to see at least someone is still paying attention to this board! It seems like it had quite the following. I just posted a fairly thorough Instablog on Seeking Alpha. and as soon as I am approved to become moderator for this board, will update the sticky section at the top with it.
Here is a link if you're curious in the meantime:
http://seekingalpha.com/instablog/27523743-point-of-return/3686746-legal-drug-war-how-vancpharma-is-taking-advantage-of-the-patent-cliff-in-canada
Yes I am still following, as I still have a lot of shares from the Alda days when Owen ran it into the ground. Here's hoping I can finally make some money with what I have left. Not selling until I am in the green or it blows up; but I must say it does sound much more promising this time.
Compelling opportunity to invest right now given:
(1) the recent pullback in share price;
(2) the significance of forthcoming news releases to act as catalysts to unlock shareholder value;
(3) management’s achievements to date and their track record to deliver results;
(4) market opportunity (>$1 billion on current portfolio) and attractive valuations of sector peers;
and (5) lack of competition in the Western Canadian generics market and the opportunity to generate imminent revenue.
Simple business model - Vanc sources drugs that have already obtained FDA equivalent approvals through affiliated companies in China and India, in exchange for manufacturing rights when the drugs are approved by Health Canada.
This has been quarterbacked by CEO Arun Nayyar, who has an extensive track record in the generics industry, having held executive positions with pharma companies in Latin America, Asia, and Canada and has played an instrumental role opening up new markets abroad
When compared to some of the other generics companies, Vancpharma looks very undervalued based solely off of its standing drug portfolio, not to mention its ability to scale quickly.
The two best comparables that come to my mind are: Paladin Labs and BioSyent.
Vanc Pharmaceuticals is nothing like the company it used to be.
Since the new management team stepped in, Vanc has completed revamped its business model and is now focusing on providing Canadian healthcare professionals and consumers with high-quality, affordable generics and over-the-counter ("OTC") healthcare products.
This company looks like its going to be a great one. I just started looking into it. It all Sounds Good!
NPH Vanc Pharma
Has anybody had a chance to visit this place in Richmond?
New Web Site: http://www.nuvapharm.com
Alda Pharmaceuticals Corp. changed to Nuva Pharmaceuticals Inc.
http://www.otcbb.com/asp/dailylist_detail.asp?d=07/25/2013&mkt_ctg=NON-OTCBB
Alda Pharmaceuticals director McCoy resigns
2012-12-05 16:12 ET - News Release
Dr. Terrance Owen reports
DR. MCCOY RESIGNS AS A DIRECTOR AND JOINS ALDA'S ADVISORY BOARD
Alda Pharmaceuticals Corp. director Dr. William McCoy has resigned, effective Nov. 28, 2012, and has joined the company's advisory board.
In 2005, Dr. McCoy became a director of Alda and chief technology officer of Phigenics LLC, a life sciences technology company based in Chicago, Ill. In 2001, he received the Intellectual Property Law Association inventor of the year award, and a medal for outstanding contribution to management and science from the International Water Association in Berlin. He has served the American Society of Heating, Refrigerating and Air-conditioning Engineers (ASHRAE) as chair of the environmental health committee, and is presently chair of the committee developing a new standard: preventing legionellosis associated with building water systems. Dr. McCoy authored a full-length book, "Preventing Legionellosis," in 2006, and holds 29 patents in diagnostics, control and manufacturing of anti-microbials. Dr. McCoy's expertise in infection control will be an important asset to the company as it continues the development of its T36 technology.
The company wishes to thank Dr. McCoy for his service as a director and welcomes him to the advisory board. He joins Dr. Brian Conway, who is an internationally recognized researcher in the field of infectious diseases. The company is expecting to appoint additional equally qualified individuals to its advisory board to reflect its focus on the acquisition of the global marketing and sales rights to novel generic, over-the-counter and natural pharmaceutical products.
The company currently has a vacancy on its board of directors and is seeking a qualified person who can contribute to this new international pharmaceutical marketing effort.
We seek Safe Harbor.
Alda Pharmaceuticals arranges $1-million financing
2012-12-03 18:46 ET - News Release
Mr. Terrance Owen reports
NON BROKERED PRIVATE PLACEMENT FINANCING
Alda Pharmaceuticals Corp. has arranged a non-brokered private placement of up to 10 million units at a price of 10 cents per unit for gross proceeds of up to $1-million.
Each unit will consist of one common share of the company and one share purchase warrant. Each warrant entitles the holder to purchase one additional common share of the company for a period of 24 months at a price of 20 cents per common share.
Any finder's fee will be paid by the company in accordance with the policies of the TSX Venture Exchange.
Proceeds from the offering will be used by Alda for general corporate and working capital purposes.
The above private placement is subject to all regulatory and board approvals.
Alda appoints Beukman, Kennedy directors
2012-11-15 18:04 ET - News Release
Mr. Terrance Owen reports
NEW DIRECTORS JOIN THE BOARD OF ALDA
Eugene Beukman and Tom Kennedy have joined the board of directors of Alda Pharmaceuticals Corp., effective immediately.
Mr. Beukman is the corporate counsel of the Pender group of companies, a position he has held since January, 1994. He graduated from the Rand University of Johannesburg, South Africa, with a bachelor of law degree and a bachelor of law honours postgraduate degree in 1987. He has over 30 years experience in the acquisition of assets and joint ventures. He is president and director of a number of publically traded companies on the TSX Venture Exchange.
Mr. Kennedy is a graduate of the University of British Columbia, having received his bachelor of commerce and business administration degree in 1973 and his juris doctor degree in 1974. Mr. Kennedy was admitted to the B.C. bar in 1975 and has practised law for 36 years. After seven years of employment with the Canadian Federal Department of Justice, Mr. Kennedy has primarily focused as a legal, financial and business consultant to publicly traded companies. Mr. Kennedy is currently a member of the Law Society of British Columbia, the Canadian Bar Association and the British Columbia Bar Association and an associate member of the American Bar Association. Mr. Kennedy currently serves in positions as chief executive officer, president, vice-president and secretary of several TSX Venture Exchange and Canadian National Stock Exchange publicly traded companies, primarily focused on mineral exploration.
Mr. Beukman and Mr. Kennedy replace Dr. Ronald Zokol and Dr. Linda Allison, who have served as directors since 2003 and have resigned from the board as of Nov. 13, 2012. The company wishes to express its gratitude to Dr. Zokol and Dr. Allison for their long service as directors and wishes them well in their future endeavours.
We seek Safe Harbor.
Dr. Owen:
I have to say that you are doing a fantastic job at growing the company since the consolidation. We are all extremely impressed with your ability to operate a company in such an exemplary manner. Bravo and keep up the great work. We are all looking forward to hearing about all of the wonderful new ideas coming down the pipeline. Good day to you sir and may life bring you nothing but happiness and good health.
*******
__________________________________________________________________
Do I sense a hint of sarcasm in your message?
Progress has not been as rapid as I had hoped. If you were in my shoes, you would see that much of what needs to be done in public companies is laying ground work and waiting. It is frustrating but comes with the territory.
Regards,
Terry Owen
Nanotech Systems apppoints Owen as Chief Executive Officer, effective May 1, 2012.
(RTTNews) - Nanotech Systems, Inc. said Tuesday that it has appointed Dr. Terrance Owen as Chief Executive Officer, effective May 1, 2012.
Dr. Owen is the co-founder and has been the President and CEO of ALDA Pharmaceuticals Corp., which has developed novel, patented, OTC infection control therapeutics for topical use, since 2000. From 1980 to 2002, he was the President of Helix Biotech , which provided DNA testing services for paternity, immigration and forensic cases across Canada. From 1995 to 1998, he was also the President of Helix BioPharma Corp.
Dr. Owen has 25 years of executive experience with publicly-traded life sciences companies in Canada and the US. He will be responsible for maintaining Nanotech's listing on the Frankfurt Stock Exchange, which is eliminating its First Quotation Board and requiring companies to meet the Entry Standard listing requirements by December 15,2012.
by RTT Staff Writer
http://www.rttnews.com/1929544/nanotech-systems-appoints-terrance-owen-as-ceo.aspx?type=qf&utm_source=google&utm_campaign=sitemap
NR on Stockhouse re: debts
So as you should see, sp back down now. This POS slime-bag Owen, is nothing but a crook. Instead of building Alda into a real company, with real products, he preferred to screw investors, and run with the $$$. He is the lowest of the low, and I hope he's reading this.
As long as Owen is in control you can be sure this has nothing to do with production or sale of any product... I should also add promotion of those products.
Darned if I know ... is there actually a location with product that is being sold and moved ... is the downtown office all there is and is it about business deals only rather than Alda's products?
Someone dropped $56,000 into Alda in April. What's going on?
Interesting, but it's not surprising to see that the Alda gang were never that focused on making Alda a success.
Here's something I found on SH, and it might be an option for Alda.
"I'm not sure if this idea is feasible, but maybe Alda could license their products to a good MLM networking company. If only the hand sanitizer itself is everything it's cracked up to be I'm sure there would be interested parties. They got the patents, FDA approval, etc. Alda dosen't have to do any of the leg work, which we know they are incapable of anyway. They would still own the brand, well receiving royalties on sales. What's wrong with this idea?"
Seems like a good solution to me
Alda gang and Fortunate Sun Mining
May 08, 2012
Fortunate Sun Mining Grants Stock Options
VANCOUVER, BRITISH COLUMBIA--( May 8th, 2012) - Fortunate Sun Mining Company Ltd. (TSX VENTURE:FSM) announces that pursuant to its stock option plan, the Company has granted incentive stock options to its directors, officers and consultants to purchase in the total of 1,725,000 common shares in the capital stock of the company, subject to regulatory approval, exercisable for a period of five years, at a price of $0.20 per share. Fortunate Sun Mining Company Ltd. is a junior mining expl... (2 KB)
May 02, 2012
Fortunate Sun Mining Board of Directors Re-Elected at Annual General Meeting
VANCOUVER CANADA: Fortunate Sun Mining Company Ltd. (TSX-V: FSM) (Fortunate Sun or, the "Company") is pleased to announce that at the Company's Annual General Meeting held on April 27, 2012 the following individuals were re-elected to the board of directors: Reg Advocaat, Peter Chen, Gary Nordin, Scott Young and Terrance Owen. In addition, Reg Advocaat has been re-appointed President & CEO of the Company and Peter Chen has been re-appointed as Chief Financial Officer. Reg Advocaat, ... (3 KB)
Fortunate Sun
Mining Company Ltd,
Suite 1502 - 1166
Alberni Street,
Vancouver, BC, V6E 3Z3
Canada
Complaining on Boards about the stock or the CEO,s is worth what is worth.......
The best way you can help..... is to write to all Presidents in the Whole World.......
Checking me out? For your information that other scam, FXIT, is run by a bunch of crooks just like Alda.
No I do not understand you Drumstick!!!
I lost $400K and I had the very best Co,s......I was using the best Broker either......So NOI do NOT understand you at all???
Alda had no money for BIG PUBLICITY and no one seem to be willing to buy is product ?????.....
Lets give it a rest to Alda......
BY the way, I noticed you are complaining as well on others stock .....is it worth it to go on and on and on ????......
You're right, I did'nt loose everything. Maybe I got a few hundred bucks left of the original 60,000 I invested. Again, there where spots when I should have bailed. But in 2009, when the Olympic deal was happening, along with the SD distribution, I thought that Alada was finally on it's way. What followed, I don't have to explain. I then came to 2 conclusions. Either these people were totally stupid, or there were other reasons for getting into the Olympics in the 1st place. That's when everything became clear. I then understood the game they were playing. Put out a positive NR, and the suckers would come running. SP then spikes, as insiders dumped. The last of these as I recall was the FDA approval. I firmly believe that this Owen crook was not stupid. This then leaves me to the conclusion that he, and his people, were screwing investors. I guess in the short term, when running a public funded company it's easier to take advantage of the cash flowing in via the market, than to actually market, and sell product. I believed in this company. I believed that considering all what was going on, H1N1, and hospital infections spelled a huge market potential for the Alda product line. There was, but unfortunately the person in charge was corrupt, and had no interest in doing the work required. Instead he preferred to do what was easiest. The cash was there, and he took it. Do you understand why I'm so pissed off?
Write to the POPE!!!- he is the first BANDIT!!!.....
I was referring to the whole crooked economic system in general. The US$ is on life support. It can't go on much longer. Even Canada is going to get hit. The system we live in is unsustainable. Just look at oil. This 1 industry makes up to 10% of the world's economy. I think it's safe to say that the majority of the environmental destruction on planet earth is related to the petrol industry. There are safe friendly alternatives to most of the products derived from the petrol industry. Just consider industrial hemp. This single plant, that has so many uses is illegal to grow in the US. This is pure insanity. There are many other examples, but my point for using the petrol industry as an example is that unfortunately the greed of those in power will not stop until they've destroyed everything. We could have free non-polluting energy today. The technology exists. So why don't we? Thousands of children starve to death every day around the world. On the other end investment bankers get 100 million dollar bonuses for stealing. We are all experiencing a repeat play of what's happened many times before. I guess this is kind of what it was like in the last days of the Roman Empire. You don't have to be a genius to see where this is all headed.
Do not understand why you are so "Resentful" toward Dr. Owen
He his not worse than the Majority of CEO's that run Co's.....
The WHOLE society has change for the worse.....greed is everywhere in every sector..... The Whole World is having problem
Me too I lost all my money but I "blame myself" for my stupidity
no use to play the "naive"card.....
I am sure you did not lost everything with Alda, not possible you invested all your money there.... to me,you do not seem to be so naive beside you had time to get out with not so great loss.....
Have a great day,
janet
I'm not interested in any stocks period. As far as I'm concerned the whole thing, the market in general is rigged. It's all about greed, with deceitful scum like this Owen prick running the show. Of course I realize that there are good companies out there like Apple for example, but even there, in many respects it's the same game. You see Janet when you honestly think about it, earning money for doing nothing dosen't make sense. Take a look at the pitiful state the US economy is in today. In my opinion this is the result of years, and years of these greedy, cheating psychos, Owen types, but on a much larger scale, who've been ripping off the system. Things got a lot worse with the internet, because it became too easy for people like me to get involved. I learned the hard way. I got into Alda 5 years ago because I liked what I saw based on the products, and what I saw as a huge market potential. Problem was that due to my naivety I've lost everything. I still believe logically Alda should have done well. Call me stupid, but I also believed that the CEO would be doing everything possible to achieve that. I know now that this was not the case to say the least. If you read the letter that Woodstock posted from the MF, you see he shows no remorse. I'm sure he feels no guilt either. So I'm not interested in any of your latest pumps, but I wish you luck in all your investment activities.
Do not know what make you say this:
<< At least 1 thing is for sure though, it won't last much longer.>> ????
If for sure there would be a change that very good because YES we do need a change for the better !!!......
I don't involve myself in the scam market anymore. I've had too many experiences with BS operations like Alda, run by slime- balls like Terry Owen. If the markets were run properly this arrogant prick would be in jail by now, but it's all rigged, and most of these crooks get rewarded instead of punished. At least 1 thing is for sure though, it won't last much longer.
In your words....Alda is so very deceiptful .....but hey, here a good stock for you:RIOCAN symbol REI.UN- Contrary to Alda "REI.UN" pay nice dividend as well.....
Sweet Dreams,
janet
I love the line where he says rather than keeping Alda moving forward. You got to admire the arrogance of this bastard. Alda already is an empty shell, and it didn't have to be this way if there'd been an honest hard working, well intentioned management. Dr.Owen, Dr. of scam.
This is in answer to my latest correspondence with Dr Owen. I received the answer to my email on the same day, which was yesterday. It does not say much; but at least he always answers. Don't know if this company will ever get back on its feet again; but until Alda is a total bust, I will continue to check in to see if its heart is still beating.
"Of course you realize that I cannot disclose anything that is not already public information.
As you know, we have a Memorandum of Understanding with a private pharmaceutical company to acquire certain of their products. Further, ALDA is still in possession of its intellectual property for which a very detailed plan is presented in the MD&A, subject to financing, of course.
Beyond that, I am not at liberty to disclose any plans, as it would be a violation of securities laws to do so.
Further, as our plans are consummated, we can only disclose what is accomplished at the time regardless of how that may fit into a larger strategy that cannot be disclosed until that also unfolds. No matter what we do, someone will be displeased but what we are doing is, in our opinion, in the best interests of shareholders.
Shareholders who contemplate "action" may not realize that, rather than keeping ALDA moving forward as a pharmaceutical company, we could have walked away and let it become an empty shell on the NEX which, at some time in the indeterminate future, may eventually have become a mining company. That alternative would have been a much longer wait than what we have pursued and I am aware of no "action" that has been successfully pursued against the principals of companies that have legitimately followed this course.
As always, I am working towards moving the company forward."
Regards,
Terry Owen
Let's face it, the whole system is rigged. It doesn't take a rocket scientist to figure out what happened to Alda. Every-time he put out a NR you'd see a brief rally, then the dumping began. I know that actual physical products existed, but there was never any marketing, or the kind of real efforts made that you'd expect from a company in this type of business. Even with the Olympic sponsorship, they failed, miserably. Then news comes out not that long ago, that Owen has a partner, Harry Chew, a fine outstanding gentleman with alleged ties to Hong Kong organized crime.
Yes I think a criminal investigation is warranted, on moral grounds, but hey, this is the Venture exchange. Probably 1/2 the companies there should be investigated. A low life, POS, slime-ball like Terence Owen might not be interested, or have the ability to build a real company, no matter how great the products, simply because it's much easier to screw the investors, and cry his tired, "market conditions" excuse, all the way to the bank. It should be much more difficult for these crooks to get a public listing.
If in fact there has been unethical behaviour rather than just an exceptional degree of incompetence (rooted in hubris, seems like), is there no recourse? Or is proof the issue?
Looks like aph has sunk into the category of a worthless shell. It's got nothing to do with the creation, distribution, and sales of physical products for which many investors were unfortunately led to believe by a small group of white collared criminals, led by Terry Owen. Maybe this is nowhere the scope of an Enron, but the inherent criminal activity that has moat likely been committed is not much different... in spirit anyway.
Alda Pharmaceuticals reinstated for trading
2012-03-09 20:19 ET - Cease Trade Company Rescinded
Further to the TSX Venture Exchange bulletins dated Nov. 4, 2011, and March 7, 2012, the exchange has been advised that the cease trade order issued by the British Columbia Securities Commission on Nov. 4, 2011, has been revoked.
Effective at the opening Monday, March 12, 2012, trading will be reinstated in the securities of Alda Pharmaceuticals Corp. (Cusip 01407W 20 5).
|
Followers
|
26
|
Posters
|
|
|
Posts (Today)
|
0
|
Posts (Total)
|
4094
|
|
Created
|
08/07/04
|
Type
|
Free
|
| Moderators | |||
INVESTMENT SUMMARY
- Received a Drug Establishment License ("DEL") and approvals from Health Canada for 30 of its 49 prescription generics products; already has manufacturing approved, labeling and packing secured, and is establishing a sales force 1Q15 salesforc
- Simple business model: Vanc sources drugs that have already obtained FDA equivalent approvals through affiliated companies in China and India, in exchange for manufacturing rights when the drugs are approved by Health Canada
- Potential to scale quickly, comparable to another Canadian company, Paladin Labs, that got bought out for $1.6 billion in 2013 by Endo Health (NASDAQ:ENDP)
- CEO, Arun Nayyar has an extensive track record in the industry - specifically generics - having held executive positions with pharma companies in Latin America, Asia, and Canada and played an instrumental role opening up new markets abroad
- Pre-revenue, albeit with all of the components in place to immediately impact the generics market in Western Canada, and unlock shareholder value through a number of visible, value-unlocking events throughout 2015
Between 2010 and August 2013, brand-name drugs with sales totaling $6 billion a year in Canada lost their patent protection - opening the door to far cheaper generic copies, according to the IMS Brogan market-research company.
Compounding the so-called "patent cliff," a growing list of insurance companies that manage private, workplace drug plans have recently made substituting generics for brands a mandatory policy - a step that most government plans took years ago. The result: more then two thirds of prescriptions in Canada are now filled with generics, while some brand manufacturers lack new drugs in the pipeline to take up the slack.
COMPANY OVERVIEW
Vanc Pharmaceuticals Inc. ("Vancpharma") (OTCQB: NUVPF) is a Canadian company (TSX-V: NPH) focused on providing Canadian health care professionals and consumers with high quality, affordable generics and over-the-counter ("OTC") healthcare products. They are the first Canadian generics company in Western Canada.
GENERICS PORTFOLIO
The company's currently approved in-licensed generics portfolio consists of 30 molecules, comprising of 67 dosage forms across various therapeutic categories: including both chronic (long-term) therapy and acute (short-term) therapy. Management estimates that the aggregate annual Canadian sales of its 30 approved products represents "a $1-billion market opportunity". Furthermore, the company plans to launch "with additional products and we will provide further updates in the coming months," said Arun.
The generics division of the company was only launched last Spring, since then the new management team has reached a number of significant milestones including:
On April 15th, 2014 Vancpharma announced that it had signed Cross Referencing Agreements ("CRA") "for prescription generic products for Canadian markets. These agreements are with three large pharmaceutical companies and cover 48 prescription generic products. The suppliers will handle manufacturing, and Vancpharma will market and sell these new product lines under its own label.
On November 18th, 2014 the company received approval via a Notice of Compliance ("NOC") from Health Canada for 22 generic molecules, comprising 51 dosage forms.
On December 10th, 2014 the company announced that it had been issued a drug establishment licence ("DEL") (licence No. 102220-A) by Health Canada. "The issuance of a drug establishment licence, along with the approval of our partner's GMP manufacturing site, is a key step towards the commercialization of our generic drug portfolio," said Arun Nayyar, CEO. The licence allows Vancpharma to import pharmaceutical products and distribute them within Canada.
This news was particularly important for the company and shareholders because: it positioned Vancpharma to become one of the only 40 companies in Canada that are licensed to manufacture current and future drugs at its GMP facility; allows Vancpharma to import from other manufacturers across the world and faces less barriers to entry; allows for importing both generic and non-generic drugs thus allowing the company to compete with other companies in branded drugs as well as their core generics business; and adds major clout when negotiating for exclusivity rights across Canada. Previously, manufacturers wouldn't commit their exclusively to Vancpharma as they were unsure if it could make good on importing and selling their products.
On December 15th, 2014 the company received an NOC from Health Canada for 7 additional generic molecules, comprising 15 dosage forms.
Lastly, on January 14th, 2015 Vancpharma placed inventory purchase orders for 30 generic molecules and expects to deliver these products 2Q 2015. "We are excited to take this important step towards commercialization and look forward to launching sales in Q2 2015. These 30 molecules represent best-selling generics in the Canadian market and our aim to provide Canadians with quality and cost-effective products is well served by them," said Arun. "Our initial marketing and outreach activities with select pharmacy customers in Western Canada have been positive and we look forward to working with our partners."
Figure 1: Generics Portfolio
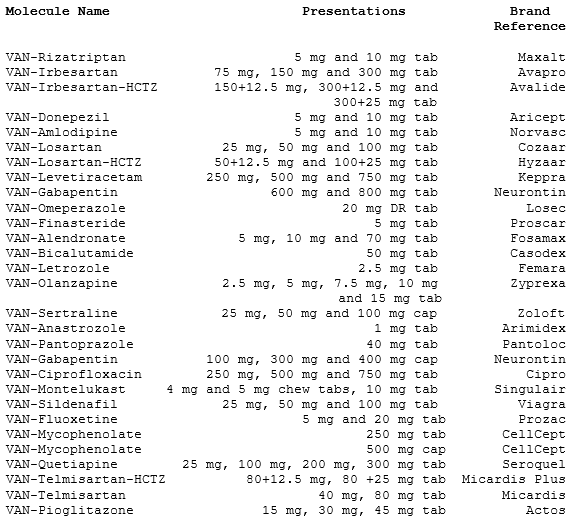
Source: Press Release/Company Website
*Note: I'm aware that's only 29, however the 30th hasn't been updated on the website although it's been mentioned as approved (new approvals could come at any time)
Here are the next steps with the generics portfolio:
Figure 2: Generics Through Global Partnership
For those wondering what Vancpharma's margins are, the company has not made that public yet. As you can imagine, they don't want to expose their margins for specific drugs too early. Investors will be able to see them in the financial statements at a later date. However, generics are quite lucrative. Vancpharma will be taking on the risk of inventory, licensing, approvals, sales and marketing, whereas the manufacturer takes and fills an order from Vancpharma as necessary and collects payment. Accordingly, the reward follows the risk and in this case, Vancpharma would pay a fixed price to the manufacturer depending on the size of the manufacturing run. If you look at the financials of another generics company, Biosyent (TSX-V: RX) you will see margins typically ~70%-75%.
The margins generated from strategic cross-reference partnerships, while very enticing, pale in comparison to the financial opportunities presented by exclusive or co-development partnerships which management has indicated they want to pursue in the future.
OTC PRODUCTS
The OTC Products Division is focused on the marketing and sales of novel and proprietary healthcare products and consists of four (4) such products, all of which now have a Natural Product Number.
Figure 3: Vancpharma Pharmaceuticals OTC Products
It can't and won't be the cash cow for Vancpharma like its generics portfolio - the Canadian market for these OTC products is only ~$60-70 million - but it should actually start generating revenue sooner. Manufacturing should commence in January, with the first sales hopefully starting to come sometime during March.
Looking ahead at the future pipeline of OTC products, they include nutraceuticals, vitamin supplements, and skin care products.
MANAGEMENT
The team has extensive experience and expertise that spans across various functions such as research, development, manufacturing, and marketing of generics and OTC health care products in the global pharmaceuticals industry. This understanding of industry best practices and strong insight allows the company to identify emerging trends in medicine and the marketplace.
The secret to being able to license so many drugs within such a short period of time, less than a year after being restructured is CEO, Arun Nayyar.
Arun only joined the company November 25th, 2013, but came with an extensive track record in the industry - specifically generics - having held executive positions with pharmaceutical companies in Latin America, Asia, and Canada. He has been instrumental in opening up new markets abroad, and domestically his accomplishments include Director, Business Development and International Sales for Shoppers Drug Mart ("SDM"), and consulting for Sanis Healthcare, George Weston Ltd. (Loblaws group), and SDM. To have a more extensive look at Arun's resume and job history, you can view his LinkedIn here.
Some information that you won't find on LinkedIn is that he's an owner of a few Shoppers Drug stores in the Vancpharmaouver area, and also has deep-ties, and solid connections in India. This helps to secure licensing of the generics in exchange for manufacturing rights when Health Canada approves the products.
The newest hire, replacing Jamie Lewin as director and CFO and announced December 3rd, 2014 was Aman Parmar. "I look forward to working with the team at Vancpharma Pharmaceuticals and am impressed by how far they have progressed with limited capital," said Mr. Parmar. "Capital efficiency and creating shareholder value will be my primary focus at Vancpharma." Since joining Vancpharma, Aman has purchased 135,000 shares on the open market ranging from 19 to 24 cents - clearly indicative that he believes the company is undervalued at these levels.
Given the fact that the company is ramping up its business, they've already started putting together a sales and marketing team, it wouldn't surprise me if another executive was brought in to help.
You can read more about the entire Vancpharma team by clicking this link (note, this page needs to be updated with Jamie/Aman).
FINANCIALS
Although Vancpharma is a pre-revenue company, I see a multiple number of visible, value-unlocking events through 2015 that can meaningfully impact stock performance.
Figure 4: Summary of Quarterly Results
The company closed an oversubscribed, non-brokered private placement for gross proceeds of $1,141,000 by issuing 7,607,332 units at $0.15/unit on December 11th, 2014. "This round of funding enables us to move our portfolio of generic drugs and OTC products into commercialization," said Arun Nayyar. "Specifically we will be acquiring generic drug inventory and building our sales team to target pharmacy customers."
The company is financed for the time being, but may have to do another round in March depending on how the roll-outs are going, and for general working capital purposes. If so, I'm sure that it would be strategic - I would think at least >$0.20, comprised primarily by sophisticated retailers and brokers, and it would not be a raise of much more than $1 million. Management only wants to raise whatever money they believe is necessary right now because they know as soon as the company starts generating revenue that its valuation has easily be many multiples of where it sits today.
SHARE STRUCTURE
Shares outstanding: 44,374,407
Stock Options: 3,975,000
Warrants: 16,212,252
Fully Diluted: 64,561,659
Major shareholders of the company, and percentage owned include:
It's a tight share structure, the 'effective' float is ~9 million shares - much less than what's been traded the past few months - 42,798,548 shares at an average price of $0.194:
Figure 5: House Positions
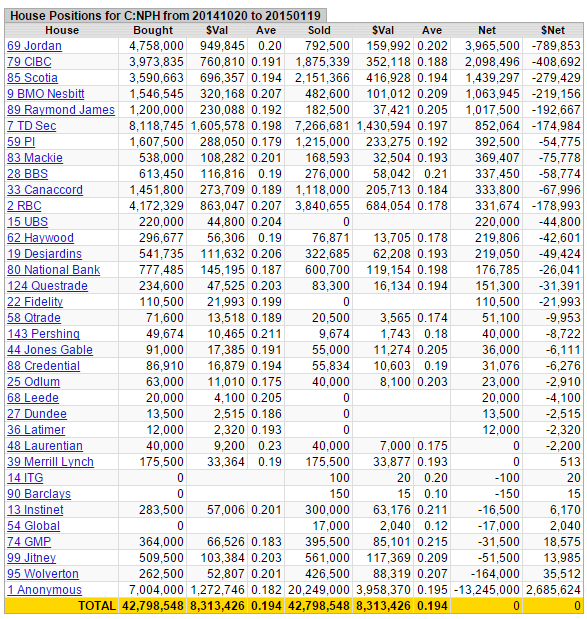
Source: Stockwatch
Figure 6: Management Insider Filings
REVENUE RAMP/VALUATION
It's a little early for me to try and assign a valuation, or forecast revenue and earnings for the company, however I anticipate doing so before year-end. The company hasn't informed investors of the specific margins and obviously we don't know the adoption rate because we don't know how good the sales team will be. Take a look at this article though, "The Top 11 Fastest-Growing Generis Companies", when compared some of the other generics companies, Vancpharma looks very undervalued based solely off of its standing drug portfolio.
The two best examples that come to my mind are: Paladin Labs and BioSyent (OTCPK:BIOYF).
Former pharma sales rep., and Cantech Letter contributor Hogan Mullally wrote that Paladin "became the poster child for Canadian specialty pharma. They built a remarkably successful business by acquiring the Canadian rights to a wide variety of drugs. These drugs were either too small for medium/big pharma, or were developed by a company without a Canadian commercial footprint, whatever the reason, Paladin was able to amass an eclectic and diverse portfolio of prescription and OTC drugs for the Canadian market. Through their "sum of the parts" strategy, Paladin grew to over $200 million in annual sales and in 2013 was acquired by Endo Pharmaceuticals for approximately $1.6 billion.
BioSyent is in between Vancpharma and Paladin, as it has already amassed an impressive portfolio by searching the globe to in-license or acquire innovative pharmaceutical products for the Canadian market. It too focuses on products that are too small for medium/big pharma, and have a competitive angle that can be exploited by a modest sales force. BioSyent's business model is structured to minimize risk, and to produce high growth. For the four years ended December 31, 2013 the company experienced a CAGR of 67% while consistently growing profits:
Figure 7: BioSyent Financials at a Glance
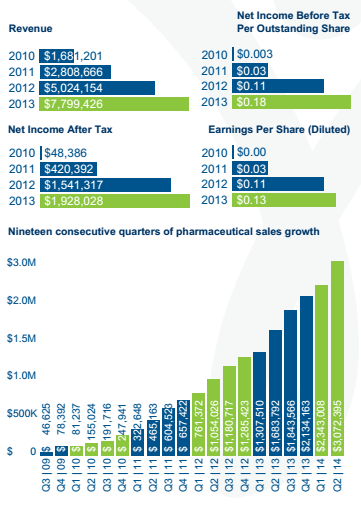
Source: Fact Sheet
There is an exit strategy for the company and shareholders - take-out target for an M&A transaction. It might take getting into a few hundred pharmacies, but the precedent has already been set. The initial adoption rate risk is reduced from the outset because between members of the management team, they own a little more than a dozen pharmacies. Not to mention now that Health Canada has granted the company a Drug Establish License it can pursue additional revenue opportunities and further de-risked the investment.
RISKS
Vancpharma is still considered an early stage company, as such there are a number of risks associated with making an investment at this point in time, including but not limited to:
(1) The company just hired a new CFO, and has a management team has dozens of years of business experience, most dealing with generic pharmaceuticals.
(2) The company plans to manufacture its products at four certified GMP pharmaceuticals factories in Canada, India and China. These U.S. FDA approved plants are capable of manufacturing a wide range of Generic Pharmaceuticals and OTC Health care products at these facilities, under the VANCPHARM label.
(3) Like I mentioned before, management controls a number of pharmacies which will de-risk the rollout process and immediately start generating revenue form the company's generics portfolio.
(4) Arun has built deep, extensive relationships in India and China, and has a lot of ideas to add more products to the portfolio (not to mention the future potential to co-development or development exclusively).
(5) The company sources its products from big products that are already approved by either the U.S. FDA or UPHRA, and also approved by Health Canada. As long as the company is in compliance with Health Canada guidelines then the ANDS application gets expedited, updated and faster tracked. The company hasn't had so much of a hiccup yet, because of the experienced team in place filing all of the paperwork.
(6) The company hasn't had an issue raising money despite the poor market for companies trading on the TSX Venture. The last PP was oversubscribed, and the shares were spread around to strong, strategic hands. Presuming management continues to achieve its milestones, I anticipate lots of eagerness for the next round.
CONCLUSION
Vancpharma set forth some ambitious goals, but has already accomplished so much in such a short amount of time that I really have large aspirations for it (and shareholders).
The company has a tried and tested business model which is simple to understand. Vancpharma enters into Cross-Referencing agreements with affiliated companies whom source products from China and India which are already approved, in exchange for manufacturing rights when the drugs are approved by Health Canada. This is very economical as there is a minimal cross-referencing fee paid to Health Canada, and a very large market opportunity (>$1 billion for current generics portfolio), with margins typically ~70%-75%.
Source: StockCharts.com
Taking a look at recent trading, there has been some healthy consolidation after the initial big run-up, ~33% off its 52-week high. MACD and RSI indicators are now in 'oversold' territory, and the stock is approaching its 200-day moving average. Up until a few months ago this stock traded 'by appointment only'. Since the middle of October, 42,798,548 shares at an average price of $0.194 have traded.
Now that the company has received 30 of its 49 Health Canada approvals (with the rest to be submitted shortly), there is a bit of work that needs to be done in order advance it from being a purely speculative growth biotech with Health Canada approvals, to one that's one being noticed by institutions and an American audience.
In the meantime, investors have two options: (1) buy now, taking the risk knowing that the company may need to finance further, but is extremely close to generating revenue; or (2) sit on the sidelines and wait until the second quarter financials are released, and make a decision then.
The latter is much less risky, however if management successfully executes on its business plan and stays on track with milestones, then I'm sure its share price will reflect it this, and it will be at a significant premium.
Bottom line, I think that there is a very compelling opportunity to invest right now given: (1) the recent pullback in share price; (2) the significance of forthcoming news releases to act as catalysts to unlock shareholder value; (3) management's achievements to date and their track record to deliver results; (4) market opportunity (>$1 billion on current portfolio) and attractive valuations of sector peers; and (5) lack of competition in the Western Canadian generics market and the opportunity to generate imminent revenue.
(For additional liquidity, NUVPF. trades in Canada on the TSX Venture as "NPH". 3-month average volume 637,531 shares/day.)
Please feel free to comment below or send me an inbox message if you have any questions or comments about this article.
| Volume | |
| Day Range: | |
| Bid Price | |
| Ask Price | |
| Last Trade Time: |
