Register for free to join our community of investors and share your ideas. You will also get access to streaming quotes, interactive charts, trades, portfolio, live options flow and more tools.
Expected another extension of the tender offer.
I was unable to meet the timeline.
HARMONY BIOSCIENCES COMPLETES ACQUISITION OF ZYNERBA PHARMACEUTICALS AND EXPANDS PIPELINE (10/11/23)
New product in development diversifies portfolio to drive long-term growth
Innovative potential new therapeutic option for rare/orphan neuropsychiatric disorders with high unmet medical needs
Zygel™ in pivotal Phase 3 trial for Fragile X syndrome and has completed Phase 2 proof-of-concept study in 22q11.2 deletion syndrome
PLYMOUTH MEETING, Pa., Oct. 11, 2023 /PRNewswire/ -- Harmony Biosciences Holdings, Inc. ("Harmony") (Nasdaq: HRMY), a pharmaceutical company dedicated to developing and commercializing innovative therapies for patients with rare neurological diseases, today announced that it has completed its acquisition of Zynerba Pharmaceuticals, Inc. ("Zynerba") (Nasdaq: ZYNE).
Zynerba is a leader in pharmaceutically produced transdermal cannabinoid therapies for orphan neuropsychiatric disorders. Harmony President & CEO Jeffrey M. Dayno, MD, described its lead candidate, Zygel, as a 'portfolio in a product' with the potential to serve 80,000 U.S. patients who are diagnosed with Fragile X syndrome (FXS) and another 80,000 diagnosed with 22q deletion syndrome (22q).
"Zygel is a significant market opportunity that advances our long-term growth strategy of developing a diversified portfolio beyond sleep/wake therapies," Dayno said. "This acquisition expands our pipeline with a product candidate in our area of expertise that could address high unmet medical needs for people living with rare neuropsychiatric disorders."
Zygel is the first-and-only pharmaceutically manufactured synthetic cannabidiol. It is a non-euphoric cannabinoid formulated as a patent-protected permeation-enhanced gel for transdermal delivery through the skin and into the circulatory system. The product is manufactured through a synthetic process in a cGMP facility and is not extracted from the cannabis plant.
Zygel does not contain THC, the compound that causes the euphoric effect of cannabis, and has the potential to be a nonscheduled product if approved.
Cannabidiol, the active ingredient in Zygel, has been granted orphan drug designation by the United States Food and Drug Administration (FDA) and the European Medicines Agency (EMA) for the treatment of FXS and for the treatment of 22q. Additionally, Zygel has received FDA Fast Track designation for the treatment of behavioral symptoms in patients with FXS.
Harmony's tender offer to acquire all outstanding shares of Zynerba for a purchase price of $1.1059 per share in cash, or $60 million in the aggregate, plus one non-tradeable contingent value right (CVR) per share, representing the right to receive potential additional payments of up to $140 million or approximately $2.5444 in additional cash per share, subject to the achievement of certain clinical, regulatory and sales milestones, expired at 5:00 p.m. New York City time on Tuesday, October 10, 2023. The depositary for the tender offer has advised that, as of the expiration of the tender offer, a total of 28,236,148 shares of Zynerba's common stock were validly tendered and not withdrawn in the tender offer, which represent approximately 52.3% of the total number of shares of Zynerba's outstanding common stock (not including 1,072,940 shares delivered through Notices of Guaranteed Delivery, representing approximately 2.0% of the shares outstanding).
Following the acceptance of the tendered shares, Harmony completed the acquisition of Zynerba through the merger of a wholly owned subsidiary of Harmony with and into Zynerba in which each share of Zynerba's common stock issued and outstanding immediately prior to consummation of the merger (other than shares (a) held in Zynerba's treasury, (b) owned by Harmony at the time the offer commenced, (c) irrevocably accepted for payment in the tender offer or (d) shares held by Zynerba stockholders who properly demanded appraisal for their shares under Delaware law), including shares granted under Zynerba's equity compensation arrangements that were not validly tendered in the tender offer, was converted into the right to receive $1.1059 per share in cash plus one CVR. As a result of the merger, Zynerba became a wholly owned subsidiary of Harmony. The common stock of Zynerba will no longer be listed for trading on the Nasdaq Capital Market.
About Harmony Biosciences
At Harmony Biosciences, we specialize in developing and delivering treatments for rare neurological diseases that others often overlook. We believe that where empathy and innovation meet, a better life can begin for people living with neurological diseases. Established by Paragon Biosciences, LLC, in 2017 and headquartered in Plymouth Meeting, PA, our team of experts from a wide variety of disciplines and experiences is driven by our shared conviction that innovative science translates into therapeutic possibilities for our patients, who are at the heart of everything we do. For more information, please visit www.harmonybiosciences.com.
About Fragile X Syndrome
Fragile X syndrome (FXS) is a rare genetic disorder that is the leading known cause of both inherited intellectual disability and autism spectrum disorder, affecting 1 in 3,600 to 4,000 males and 1 in 4,000 to 6,000 females. The disorder negatively affects synaptic function, plasticity and neuronal connections, and results in a spectrum of intellectual disabilities and behavioral symptoms, such as social avoidance and irritability. There are approximately 80,000 people in the U.S. and approximately 121,000 people in the European Union and UK living with FXS. There is a significant unmet medical need in patients living with FXS as there are currently no FDA approved treatments for this disorder.
FXS is caused by a mutation in FMR1, a gene which modulates a number of systems, including the endocannabinoid system, and most critically, codes for a protein called FMRP. The FMR1 mutation manifests as multiple repeats of a DNA segment, known as the CGG triplet repeat, resulting in deficiency or lack of FMRP. FMRP helps regulate the production of other proteins and plays a role in the development of synapses, which are critical for relaying nerve impulses, and in regulating synaptic plasticity. In people with full mutation of the FMR1 gene, the CGG segment is repeated more than 200 times, and in most cases causes the gene to not function. Methylation of the FMR1 gene also plays a role in determining functionality of the gene. In approximately 60% of patients with FXS, who have complete methylation of the FMR1 gene, no FMRP is produced, resulting in dysregulation of the systems modulated by FMRP.
About 22q11.2 Deletion Syndrome
22q11.2 deletion syndrome (22q) is a disorder caused by a small missing piece of the 22nd chromosome. The deletion occurs near the middle of the chromosome at a location designated q11.2. It is considered a mid-line condition, with physical symptoms including characteristic palate abnormalities, heart defects, immune dysfunction, and esophageal / GI issues, as well as debilitating neuropsychiatric and behavioral symptoms, including anxiety, social withdrawal, ADHD, cognitive impairment and autism spectrum disorder. It is estimated that 22q occurs in one in 4,000 live births, suggesting that there are approximately 80,000 people living with 22q in the U.S. and 129,000 in the European Union and the UK. Patients with 22q deletion syndrome are managed by multidisciplinary care providers, and there are currently no FDA approved treatments for this disorder.
https://www.prnewswire.com/news-releases/harmony-biosciences-completes-acquisition-of-zynerba-pharmaceuticals-and-expands-pipeline-301953012.html
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
SCHEDULE 14D-9
(Rule 14d-101)
(Amendment No. 5)
SOLICITATION/RECOMMENDATION STATEMENT
UNDER SECTION 14(d)(4) OF THE SECURITIES EXCHANGE ACT OF 1934
Zynerba Pharmaceuticals, Inc.
(Name of Subject Company)
Zynerba Pharmaceuticals, Inc.
(Name of Persons Filing Statement)
COMMON STOCK, PAR VALUE $0.001 PER SHARE
(Title of Class of Securities)
98986X109
(CUSIP Number of Class of Securities)
Armando Anido
Zynerba Pharmaceuticals, Inc.
Chairman and Chief Executive Officer
80 W. Lancaster Avenue, Suite 300
Devon, Pennsylvania 19333
(484) 581-7505
(Name, address, and telephone numbers of person authorized to receive notices and communications
on behalf of the persons filing statement)
With copies to:
Rachael M. Bushey, Esq.
Jennifer L. Porter, Esq.
Laura K. Umbrecht, Esq.
Goodwin Procter LLP
One Commerce Square
2005 Market St., 32nd Floor
Philadelphia, PA 19103
(445) 207-7800
?
Check the box if the filing relates solely to preliminary communications made before the commencement of a tender offer.
Explanatory Note:
This Amendment No. 5 (this “Amendment”) amends and supplements the Solicitation/Recommendation Statement on Schedule 14D-9 (as amended or supplemented from time to time, the “Schedule 14D-9”) previously filed by Zynerba Pharmaceuticals, Inc., a Delaware corporation (“Zynerba” or the “Company”), with the Securities and Exchange Commission (the “SEC”) on August 28, 2023, relating to the tender offer by Xylophone Acquisition Corp., a Delaware corporation (“Purchaser”), a wholly owned subsidiary of Harmony Biosciences Holdings, Inc., a Delaware corporation (“Harmony Biosciences”), to acquire all of the issued and outstanding shares of Zynerba’s common stock, par value $0.001 per share (the “Shares”) for (i) $1.1059 per Share in cash, subject to any applicable withholding of taxes and without interest, plus (ii) one contingent value right per Share, subject to any applicable withholding of taxes and without interest, upon the terms and subject to the conditions set forth in the Offer to Purchase, filed by Harmony Biosciences and Purchaser with the SEC on August 28, 2023 (as amended or supplemented from time to time, the “Offer to Purchase”), and in the related Letter of Transmittal filed by Harmony Biosciences and Purchaser with the SEC on August 28, 2023 (as amended or supplemented from time to time, the “Letter of Transmittal,” which, together with the Offer to Purchase, constitute the “Offer”).
All page references used herein refer to pages in the Schedule 14D-9 before any additions or deletions resulting from the supplemental disclosures, and capitalized terms used herein, unless otherwise defined, have the meanings set forth in the Schedule 14D-9. Underlined and bolded text shows text being added to a referenced disclosure in the Schedule 14D-9 and stricken-through text shows text being deleted from a referenced disclosure in the Schedule 14D-9. Except as specifically noted herein, the information set forth in the Schedule 14D-9, as amended by the Solicitation/Recommendation Statement (Amendment No. 1) on Schedule 14D-9 filed with the SEC on September 14, 2023, the Solicitation/Recommendation Statement (Amendment No. 2) on Schedule 14D-9 filed with the SEC on September 15, 2023, the Solicitation/Recommendation Statement (Amendment No. 3) on Schedule 14D-9 filed with the SEC on September 27, 2023 and the Solicitation/Recommendation Statement (Amendment No. 4) on Schedule 14D-9 filed with the SEC on September 29, 2023, remains unchanged. This Amendment is being filed to reflect certain updates as set forth below.
ITEM 2.
IDENTITY AND BACKGROUND OF FILING PERSON
Item 2 of the Schedule 14D-9 is hereby amended and supplemented as follows:
The disclosure under the heading “Tender Offer” is hereby amended and supplemented by replacing the third full paragraph beginning on page 2 of the Schedule 14D-9 in its entirety with the following:
The Offer is described in a Tender Offer Statement on Schedule TO (as amended or supplemented from time to time, and together with the exhibits thereto, the “Schedule TO”), filed by Harmony Biosciences and Purchaser with the U.S. Securities and Exchange Commission (the “SEC”) on August 28, 2023. Copies of the Offer to Purchase, and form of Letter of Transmittal and form of Notice of Guaranteed Delivery are filed as Exhibits (a)(1)(A), and (a)(1)(B) and (a)(1)(F) hereto, respectively, and are incorporated herein by reference. The Offer to Purchase and form of Letter of Transmittal are being mailed to Zynerba’s stockholders together with this Schedule 14D-9.
The disclosure under the heading “Tender Offer” is hereby amended and supplemented by replacing the second full bullet point beginning on page 3 of the Schedule 14D-9 in its entirety with the following:
•
Each restricted stock award of the Company subject to vesting conditions based solely on continued employment or service to the Company and its subsidiary (a “Company RSA”) (x) that is outstanding immediately prior to the Effective Time and (y) of which the underlying shares of common stock of the Company are not validly tendered in the Offer, whether vested or unvested, will be cancelled and automatically converted into the right to receive for each Share subject to a Company RSA, without interest and subject to deduction for any required withholding under applicable tax law, (i) an amount in cash equal to the Closing Amount and (ii) one CVR.
The disclosure under the heading “Tender Offer” is hereby amended and supplemented by replacing the second full paragraph beginning on page 3 of the Schedule 14D-9 in its entirety with the following:
The obligation of Purchaser to purchase the Shares tendered in the Offer is subject to the satisfaction or waiver of a number of conditions set forth in Annex I to the Merger Agreement, including (i) that there will have been validly tendered (and “received” as defined in Section 251(h) of the DGCL) and not validly withdrawn prior to the expiration date of the Offer (but excluding Shares tendered pursuant to guaranteed delivery procedures that have not yet been “received”, as defined by Section 251(h)(6)(f) of the DGCL by the “depository” (as such term is defined in Section 251(h)(6)(c) of the DGCL)); (ii) that number of Shares that, considered together with all other Shares (if any) beneficially owned by Harmony Biosciences and its controlled affiliates, represent one more than 50% of the total number of Shares outstanding at the time of the expiration of the Offer (the “Minimum Condition”); and (iii) those other conditions set forth in Annex I to the Merger Agreement and further summarized in Section 15 of the Offer to Purchase (collectively, the “Offer Conditions”).
ITEM 3.
PAST CONTACTS, TRANSACTIONS, NEGOTIATIONS AND AGREEMENTS
Item 3 of the Schedule 14D-9 is hereby amended and supplemented as follows:
The disclosure under the heading “Arrangements Between Zynerba and its Executive Officers, Directors and Affiliates” is hereby amended and supplemented by replacing the third full bullet point beginning on page 7 of the Schedule 14D-9 in its entirety with the following:
•
Each Company RSA (x) that is outstanding immediately prior to the Effective Time and (y) of which the underlying shares of common stock of the Company are not validly tendered in the Offer, whether vested or unvested, will be cancelled and automatically converted into the right to receive, for each Share subject to such Company RSA, without interest and subject to deduction for any required withholding under applicable tax law, (A) an amount in cash from Harmony Biosciences or the Surviving Corporation equal to the Closing Amount and (B) one CVR.
The disclosure under the heading “Consideration Payable for Outstanding Shares and Company Options Pursuant to the Offer or the Merger” is hereby amended and supplemented by replacing the second full paragraph beginning on page 7 of the Schedule 14D-9 in its entirety with the following:
Immediately prior to the Effective Time, by virtue of the Merger and without any action on the part of any holder thereof, each Company Option and each Company RSA (of which the underlying shares of common stock of the Company are not validly tendered in the Offer), whether vested or unvested, that is outstanding immediately prior thereto, will be cancelled and converted into the right to receive the amount, if any, described above in the section entitled “—Effect of the Transactions on Company Equity Awards.”
ITEM 4.
THE SOLICITATION OR RECOMMENDATION
Item 4 of the Schedule 14D-9 is hereby amended and supplemented as follows:
The disclosure under the heading “Intent to Tender” is hereby amended and supplemented by replacing the third full paragraph of that section beginning on page 27 of the Schedule 14D-9 in its entirety with the following:
To Zynerba’s knowledge, after making reasonable inquiry, all of Zynerba’s executive officers and directors currently intend to tender or cause to be tendered pursuant to the Offer all of their Shares (other than unvested Company RSAs) held of record or beneficially owned by such persons immediately prior to the expiration of the Offer, as it may be extended (other than Shares for which such holder does not have discretionary authority). The foregoing does not include any Shares over which, or with respect to which, any such executive officer or director acts in a fiduciary or representative capacity or is subject to the instructions of a third party with respect to such tender.
ITEM 8.
ADDITIONAL INFORMATION
Item 8 of the Schedule 14D-9 is hereby amended and supplemented as follows:
The following new subsection shall be added immediately before the final subsection entitled “Cautionary Note Regarding Forward-Looking Statements”:
Amendment No. 1 to Merger Agreement
On October 4, 2023, Zynerba, Purchaser and Harmony Biosciences entered into Amendment No. 1 to the Merger Agreement, which is filed as Exhibit (e)(28) to this Schedule 14D-9 and is incorporated herein by reference, in connection with changes made to the Company RSAs by the Company to permit holders of unvested Company RSAs to tender such shares of common stock of the Company underlying those unvested Company RSAs in the Offer.
The subsection of Item 8 of the Schedule 14D-9 entitled “Extension of Offer Expiration Date” is amended and supplemented by adding the following paragraph at the end of this subsection:
On October 4, 2023, Zynerba issued a press release reminding its stockholders to tender their Shares to Purchaser pursuant to the Offer before 5:00 p.m. (New York City time) on October 10, 2023. A copy of the press release is filed as Exhibit (a)(5)(N) to the Schedule 14D-9 and is incorporated herein by reference.
ITEM 9.
EXHIBITS
Item 9 of the Schedule 14D-9 is hereby amended and supplemented by adding the following Exhibits to the list of Exhibits:
Exhibit
No.
Description
(a)(1)(F)
Form of Notice of Guaranteed Delivery (incorporated herein by reference to Exhibit (a)(1)(G) to the Schedule TO).
(e)(28)
Amendment No. 1 to Agreement and Plan of Merger, dated as of October 4, 2023, by and among Harmony Biosciences Holdings, Inc., Xylophone Acquisition Corp. and Zynerba Pharmaceuticals, Inc. (incorporated herein by reference Exhibit (d)(5) to the Schedule TO Amendment No. 3).
(a)(5)(N)*
Press Release issued by Zynerba Pharmaceuticals, Inc., dated October 4, 2023.
*
Filed herewith.
SIGNATURE
After due inquiry and to the best of my knowledge and belief, I certify that the information set forth in this statement is true, complete and correct.
Date: October 4, 2023
Zynerba Pharmaceuticals, Inc.
By:
/s/ Armando Anido
Name:
?Armando Anido
Title
?Chairman and Chief Executive Officer
Exhibit (a)(5)(N)
Important Information for Zynerba Pharmaceuticals Stockholders to Tender Shares for the Harmony Transaction: Process and Instructions
DEVON, Pa., October 4, 2023 – Zynerba Pharmaceuticals, Inc. (Nasdaq: ZYNE), the leader in innovative pharmaceutically-produced transdermal cannabinoid therapies for orphan neuropsychiatric disorders, today reminds all stockholders, including individual stockholders, to promptly tender their shares into the tender offer (the “Offer”) by Harmony Biosciences Holdings, Inc. (“Harmony”) and its wholly owned subsidiary, Xylophone Acquisition Corp. (“Purchaser”), to purchase all outstanding shares of Zynerba common stock. The Zynerba Board of Directors unanimously recommends that Zynerba stockholders tender their shares pursuant to the Offer.
Key Information to Know
Tender Deadline:
•
Zynerba stockholders must tender their shares by 5:00 p.m., New York City time, on Tuesday, October 10, 2023. Please note that if you hold shares of Zynerba common stock through a broker or other nominee, they may have a processing cutoff that is prior to the tender deadline (e.g., October 5th or 6th), so it is important that you act now if you want to tender your shares.
How to Tender Your Shares:
•
If you hold shares of Zynerba common stock through a broker, dealer, commercial bank, trust company or other nominee, you must instruct such broker or other nominee to tender your shares. Please contact your broker or other nominee promptly to allow sufficient time to tender. Do not wait until the tender deadline to tender your shares as they may not be counted. Please note that, in some cases, your broker's or other nominee’s processing cutoff date may have changed since the Offer deadline was first announced, so take prompt action today to ensure your shares are properly tendered prior to any applicable processing cutoff.
o
Contact Information for Commonly Used Brokers:
◾
Call TD Ameritrade at 888-723-8504, option 1
◾
Call Fidelity at 800-343-3548
◾
Call E-Trade at 1-800-387-2331
◾
Contact Robinhood at https://robinhood.com/contact
1
•
Please include the stock symbol for the offer (ZYNE) and the number of shares you would like to tender.
◾
Call Charles Schwab at 1-800-435-4000
◾
Call Morgan Stanley at 1-888-454-3965
◾
Call JP Morgan at 1-800-935-9935
◾
Call Merrill, a Bank of America Company at 1-800-637-7455
o
If your broker is not listed above, please contact your broker’s customer service department and ask to speak with Corporate Actions. From there, you should be directed to someone who can help you.
•
Zynerba stockholders should contact MacKenzie Partners, Inc., toll free by phone at 800-322-2885 or by email to tenderoffer@mackenziepartners.com with any questions or to request assistance with tendering shares.
Why You Should Tender Your Shares:
•
Every share tendered by stockholders is important.
•
If less than a majority of the outstanding shares of Zynerba common stock are tendered, the Offer will not be completed and Zynerba will not be acquired by Harmony.
•
If Zynerba is not acquired by Harmony, there are significant risks associated with Zynerba’s ability to raise the required capital to continue as a standalone company and remain listed on the Nasdaq stock market.
If the Offer is completed, stockholders will receive an up-front purchase price of $1.1059 per share plus one non-tradeable contingent value right (“CVR”) per share representing the right to receive up to an additional approximately $2.5444 per share in cash, without interest and less any applicable tax withholding, upon the achievement of certain clinical and commercial milestones related to Zygel. If all such milestones are achieved, the total consideration payable to stockholders is up to $3.6503 per share in cash when combined with the up-front purchase price. Additional transaction details and the Company’s previous Offer reminder can be found HERE.
Questions About How to Tender Shares:
•
Please contact MacKenzie Partners, Inc., toll free by phone at 800-322-2885 or by email to tenderoffer@mackenziepartners.com. They will be able to walk you through the tender process and assist with any questions.
2
•
If you hold shares of Zynerba common stock through a broker or nominee, you should contact your broker or nominee as soon as possible to answer your questions and to allow sufficient time to tender.
Cautionary Note on Forward-Looking Statements
This press release contains forward-looking statements within the meaning of The Private Securities Litigation Reform Act of 1995. We may, in some cases, use terms such as “predicts,” “believes,” “potential,” “proposed,” “continue,” “estimates,” “anticipates,” “expects,” “plans,” “intends,” “may,” “could,” “might,” “will,” “should” or other words that convey uncertainty of future events or outcomes to identify these forward-looking statements. Such statements are subject to numerous important factors, risks and uncertainties that may cause actual events or results to differ materially from Zynerba’s current expectations. Management’s expectations and, therefore, any forward-looking statements in this press release could also be affected by risks and uncertainties relating to a number of other factors, including the following: Zynerba’s cash and cash equivalents may not be sufficient to support its operating plan for as long as anticipated; Zynerba’s expectations, projections and estimates regarding expenses, future revenue, capital requirements, incentive and other tax credit eligibility, collectability and timing, and availability of and the need for additional financing; Zynerba’s ability to obtain additional funding to support its clinical development programs; the results, cost and timing of Zynerba’s clinical development programs, including any delays to such clinical trials relating to enrollment or site initiation; clinical results for Zynerba’s product candidates may not be replicated or continue to occur in additional trials and may not otherwise support further development in a specified indication or at all; actions or advice of the U.S. Food and Drug Administration, the European Medicines Agency and other foreign regulatory agencies may affect the design, initiation, timing, continuation and/or progress of clinical trials or result in the need for additional clinical trials; Zynerba’s ability to obtain and maintain regulatory approval for its product candidates, and the labeling under any such approval; Zynerba’s reliance on third parties to assist in conducting pre-clinical and clinical trials for its product candidates; delays, interruptions or failures in the manufacture and supply of Zynerba’s product candidates and Zynerba’s ability to commercialize its product candidates; the size and growth potential of the markets for Zynerba’s product candidates, and Zynerba’s ability to service those markets; Zynerba’s ability to develop sales and marketing capabilities, whether alone or with potential future collaborators; the rate and degree of market acceptance of Zynerba’s product candidates; Zynerba’s expectations regarding its ability to obtain and adequately maintain sufficient intellectual property protection for its product candidates; the extent to which health epidemics and other outbreaks of communicable diseases, including COVID-19, could disrupt our operations or adversely affect our business and financial condition; and the extent to which inflation, banking stability or global instability, including political instability, may disrupt our business operations or our financial condition. This list is not exhaustive and these and other risks are described in Zynerba’s periodic reports, including the annual report on Form 10-K, quarterly reports on Form 10-Q and current reports on Form 8-K, filed with or furnished to the Securities and Exchange Commission (the “SEC”) and available at www.sec.gov. Any forward-looking statements that Zynerba makes in this press release speak only as of the date of this press release. Zynerba assumes no obligation to update forward-looking statements whether as a result of new information, future events or otherwise, after the date of this press release.
3
Additional Information about the Acquisition and Where to Find It
This document is for informational purposes only and is neither an offer to purchase nor a solicitation of an offer to sell shares of Zynerba, nor is it a substitute for the tender offer materials that Harmony and Purchaser filed with the SEC upon commencement of the tender offer. Harmony and Purchaser initially filed tender offer materials on Schedule TO on August 28, 2023, and Zynerba initially filed a Solicitation/Recommendation Statement on Schedule 14D-9 with the SEC with respect to the tender offer on August 28, 2023. Holders of shares of Zynerba common stock are urged to read the tender offer materials (including an Offer to Purchase, a related Letter of Transmittal and certain other tender offer documents) and the Solicitation/Recommendation Statement (as each may be amended or supplemented from time to time) because they contain important information that holders of shares of Zynerba common stock should consider before making any decision regarding tendering their shares. The Offer to Purchase, the related Letter of Transmittal and certain other tender offer documents, as well as the Solicitation/Recommendation Statement, have been available to all holders of shares of Zynerba at no expense to them. The tender offer materials and the Solicitation/Recommendation Statement are available for free at the SEC’s website at www.sec.gov. In addition, these materials are available at no charge on the Enhanced SEC Filings section of the Investor Relations page of Zynerba’s website at https://www.zynerba.com/ and by directing a request to the information agent for the tender offer, MacKenzie Partners, Inc., who can be reached toll free by phone at 800-322-2885 or by email to tenderoffer@mackenziepartners.com.
Zynerba Contacts
Peter Vozzo
ICR Westwicke
Office: 443.213.0505
Cell: 443.377.4767
Peter.Vozzo@Westwicke.com
Important Information for Zynerba Pharmaceuticals Stockholders to Tender Shares for the Harmony Transaction: Process and Instructions
Source: GlobeNewswire Inc.
Zynerba Pharmaceuticals, Inc. (Nasdaq: ZYNE), the leader in innovative pharmaceutically-produced transdermal cannabinoid therapies for orphan neuropsychiatric disorders, today reminds all stockholders, including individual stockholders, to promptly tender their shares into the tender offer (the “Offer”) by Harmony Biosciences Holdings, Inc. (“Harmony”) and its wholly owned subsidiary, Xylophone Acquisition Corp. (“Purchaser”), to purchase all outstanding shares of Zynerba common stock. The Zynerba Board of Directors unanimously recommends that Zynerba stockholders tender their shares pursuant to the Offer.
Key Information to Know
Tender Deadline:
Zynerba stockholders must tender their shares by 5:00 p.m., New York City time, on Tuesday, October 10, 2023. Please note that if you hold shares of Zynerba common stock through a broker or other nominee, they may have a processing cutoff that is prior to the tender deadline (e.g., October 5th or 6th), so it is important that you act now if you want to tender your shares.
How to Tender Your Shares:
If you hold shares of Zynerba common stock through a broker, dealer, commercial bank, trust company or other nominee, you must instruct such broker or other nominee to tender your shares. Please contact your broker or other nominee promptly to allow sufficient time to tender. Do not wait until the tender deadline to tender your shares as they may not be counted. Please note that, in some cases, your broker's or other nominee’s processing cutoff date may have changed since the Offer deadline was first announced, so take prompt action today to ensure your shares are properly tendered prior to any applicable processing cutoff.
Contact Information for Commonly Used Brokers:
Call TD Ameritrade at 888-723-8504, option 1
Call Fidelity at 800-343-3548
Call E-Trade at 1-800-387-2331
Contact Robinhood at https://robinhood.com/contact
Please include the stock symbol for the offer (ZYNE) and the number of shares you would like to tender.
Call Charles Schwab at 1-800-435-4000
Call Morgan Stanley at 1-888-454-3965
Call JP Morgan at 1-800-935-9935
Call Merrill, a Bank of America Company at 1-800-637-7455
If your broker is not listed above, please contact your broker’s customer service department and ask to speak with Corporate Actions. From there, you should be directed to someone who can help you.
Zynerba stockholders should contact MacKenzie Partners, Inc., toll free by phone at 800-322-2885 or by email to tenderoffer@mackenziepartners.com with any questions or to request assistance with tendering shares.
Why You Should Tender Your Shares:
Every share tendered by stockholders is important.
If less than a majority of the outstanding shares of Zynerba common stock are tendered, the Offer will not be completed and Zynerba will not be acquired by Harmony.
If Zynerba is not acquired by Harmony, there are significant risks associated with Zynerba’s ability to raise the required capital to continue as a standalone company and remain listed on the Nasdaq stock market.
If the Offer is completed, stockholders will receive an up-front purchase price of $1.1059 per share plus one non-tradeable contingent value right (“CVR”) per share representing the right to receive up to an additional approximately $2.5444 per share in cash, without interest and less any applicable tax withholding, upon the achievement of certain clinical and commercial milestones related to Zygel. If all such milestones are achieved, the total consideration payable to stockholders is up to $3.6503 per share in cash when combined with the up-front purchase price. Additional transaction details and the Company’s previous Offer reminder can be found HERE.
Questions About How to Tender Shares:
Please contact MacKenzie Partners, Inc., toll free by phone at 800-322-2885 or by email to tenderoffer@mackenziepartners.com. They will be able to walk you through the tender process and assist with any questions.
If you hold shares of Zynerba common stock through a broker or nominee, you should contact your broker or nominee as soon as possible to answer your questions and to allow sufficient time to tender.
Cautionary Note on Forward-Looking Statements
This press release contains forward-looking statements within the meaning of The Private Securities Litigation Reform Act of 1995. We may, in some cases, use terms such as “predicts,” “believes,” “potential,” “proposed,” “continue,” “estimates,” “anticipates,” “expects,” “plans,” “intends,” “may,” “could,” “might,” “will,” “should” or other words that convey uncertainty of future events or outcomes to identify these forward-looking statements. Such statements are subject to numerous important factors, risks and uncertainties that may cause actual events or results to differ materially from Zynerba’s current expectations. Management’s expectations and, therefore, any forward-looking statements in this press release could also be affected by risks and uncertainties relating to a number of other factors, including the following: Zynerba’s cash and cash equivalents may not be sufficient to support its operating plan for as long as anticipated; Zynerba’s expectations, projections and estimates regarding expenses, future revenue, capital requirements, incentive and other tax credit eligibility, collectability and timing, and availability of and the need for additional financing; Zynerba’s ability to obtain additional funding to support its clinical development programs; the results, cost and timing of Zynerba’s clinical development programs, including any delays to such clinical trials relating to enrollment or site initiation; clinical results for Zynerba’s product candidates may not be replicated or continue to occur in additional trials and may not otherwise support further development in a specified indication or at all; actions or advice of the U.S. Food and Drug Administration, the European Medicines Agency and other foreign regulatory agencies may affect the design, initiation, timing, continuation and/or progress of clinical trials or result in the need for additional clinical trials; Zynerba’s ability to obtain and maintain regulatory approval for its product candidates, and the labeling under any such approval; Zynerba’s reliance on third parties to assist in conducting pre-clinical and clinical trials for its product candidates; delays, interruptions or failures in the manufacture and supply of Zynerba’s product candidates and Zynerba’s ability to commercialize its product candidates; the size and growth potential of the markets for Zynerba’s product candidates, and Zynerba’s ability to service those markets; Zynerba’s ability to develop sales and marketing capabilities, whether alone or with potential future collaborators; the rate and degree of market acceptance of Zynerba’s product candidates; Zynerba’s expectations regarding its ability to obtain and adequately maintain sufficient intellectual property protection for its product candidates; the extent to which health epidemics and other outbreaks of communicable diseases, including COVID-19, could disrupt our operations or adversely affect our business and financial condition; and the extent to which inflation, banking stability or global instability, including political instability, may disrupt our business operations or our financial condition. This list is not exhaustive and these and other risks are described in Zynerba’s periodic reports, including the annual report on Form 10-K, quarterly reports on Form 10-Q and current reports on Form 8-K, filed with or furnished to the Securities and Exchange Commission (the “SEC”) and available at www.sec.gov. Any forward-looking statements that Zynerba makes in this press release speak only as of the date of this press release. Zynerba assumes no obligation to update forward-looking statements whether as a result of new information, future events or otherwise, after the date of this press release.
Additional Information about the Acquisition and Where to Find It
This document is for informational purposes only and is neither an offer to purchase nor a solicitation of an offer to sell shares of Zynerba, nor is it a substitute for the tender offer materials that Harmony and Purchaser filed with the SEC upon commencement of the tender offer. Harmony and Purchaser initially filed tender offer materials on Schedule TO on August 28, 2023, and Zynerba initially filed a Solicitation/Recommendation Statement on Schedule 14D-9 with the SEC with respect to the tender offer on August 28, 2023. Holders of shares of Zynerba common stock are urged to read the tender offer materials (including an Offer to Purchase, a related Letter of Transmittal and certain other tender offer documents) and the Solicitation/Recommendation Statement (as each may be amended or supplemented from time to time) because they contain important information that holders of shares of Zynerba common stock should consider before making any decision regarding tendering their shares. The Offer to Purchase, the related Letter of Transmittal and certain other tender offer documents, as well as the Solicitation/Recommendation Statement, have been available to all holders of shares of Zynerba at no expense to them. The tender offer materials and the Solicitation/Recommendation Statement are available for free at the SEC’s website at www.sec.gov. In addition, these materials are available at no charge on the Enhanced SEC Filings section of the Investor Relations page of Zynerba’s website at https://www.zynerba.com/ and by directing a request to the information agent for the tender offer, MacKenzie Partners, Inc., who can be reached toll free by phone at 800-322-2885 or by email to tenderoffer@mackenziepartners.com.
Zynerba Contacts
Peter Vozzo
ICR Westwicke
Office: 443.213.0505
Cell: 443.377.4767
Peter.Vozzo@Westwicke.com
Primary Logo
Hello blue skies$$$$$$$$$$$$$$$$$$$
HARMONY BIOSCIENCES TO ACQUIRE ZYNERBA PHARMACEUTICALS, INC.
Acquisition expands pipeline and diversifies portfolio to drive long-term growth
Innovative potential new therapeutic option for rare/orphan neuropsychiatric disorders with high unmet medical needs
Lead asset in pivotal Phase 3 trial for Fragile X syndrome and has completed Phase 2 proof-of-concept study in 22q11.2 deletion syndrome
Conference call and webcast to be held today at 8:30 AM ET
PLYMOUTH MEETING, Pa. and DEVON, Pa., Aug. 14, 2023 /PRNewswire/ -- Harmony Biosciences Holdings, Inc. ("Harmony") (Nasdaq: HRMY), a pharmaceutical company dedicated to developing and commercializing innovative therapies for patients with rare neurological diseases, today announced a definitive agreement to acquire Zynerba Pharmaceuticals, Inc. ("Zynerba") (Nasdaq: ZYNE), a leader in innovative pharmaceutically-produced transdermal cannabinoid therapies for orphan neuropsychiatric disorders, including Fragile X syndrome (FXS).
Under the terms of the definitive agreement, Harmony will commence a tender offer to acquire all outstanding shares of Zynerba for a purchase price of $1.1059 per share in cash, or $60 million in the aggregate, plus one non-tradeable contingent value right (CVR) per share, representing the right to receive potential additional payments of up to $140 million in the aggregate, subject to the achievement of certain clinical, regulatory and sales milestones, as described in more detail below.
"This is an important step in Harmony's strategy to build a diversified portfolio of innovative assets to address unmet medical needs and drive our long-term growth. This acquisition affords us the opportunity to advance the development and delivery of a potentially transformative treatment for the symptoms of Fragile X syndrome and other rare neuropsychiatric disorders," said Jeffrey M. Dayno, M.D., President and Chief Executive Officer at Harmony Biosciences. "In addition to the strength of our core business in narcolepsy and our current life cycle management programs, led by idiopathic hypersomnia, we are excited to continue to diversify our portfolio beyond sleep/wake by adding Zynerba's clinical development programs to our pipeline. The team at Zynerba has been dedicated to these programs and we are confident that our combined efforts could have a profound impact on individuals living with rare neuropsychiatric disorders and their families."
"Harmony's development and commercial expertise, technologies, people and focus on rare neurological diseases are an excellent strategic fit with Zynerba," said Armando Anido, Chairman and Chief Executive Officer of Zynerba. "I am very proud of Zynerba's accomplishments with Zygel™ to date. With Harmony's scale, resources and proven commercial excellence, they are well positioned to potentially bring to market the first pharmaceutical product indicated for the treatment of behavioral symptoms of Fragile X syndrome and to maximize the value of Zygel."
Zynerba's lead asset, Zygel, is the first and only pharmaceutically manufactured, synthetic cannabidiol, a non-euphoric cannabinoid, formulated as a patent-protected permeation-enhanced gel for transdermal delivery through the skin and into the circulatory system. Zygel is manufactured through a synthetic process in a cGMP facility and is not extracted from the cannabis plant. Therefore, it is devoid of THC, which is what causes the euphoric effect of cannabis, and has the potential to be a nonscheduled product if approved. Zygel is currently being evaluated in a pivotal Phase 3 clinical trial for patients living with FXS, known as the RECONNECT Trial. Additionally, Zygel showed positive signals in an open label Phase 2 trial in patients living with 22q11.2 deletion syndrome (22q), called the INSPIRE Trial.
Cannabidiol, the active ingredient in Zygel, has been granted orphan drug designation by the United States Food and Drug Administration (FDA) and the European Medicines Agency (EMA) for the treatment of FXS and for the treatment of 22q. Additionally, Zygel has received FDA Fast Track designation for the treatment of behavioral symptoms in patients with FXS.
FXS is a rare genetic disorder that affects approximately 80,000 people in the U.S., causing intellectual disabilities and behavioral challenges. Despite considerable progress in medical science, there remains a significant unmet medical need in treating patients living with this debilitating disorder. There are currently no FDA approved therapies to treat FXS.
It is estimated that there are approximately 80,000 people living with 22q in the U.S. Patients with 22q are affected by symptoms related to many organ systems including neuropsychiatric symptoms such as anxiety and behavioral difficulties. There are currently no FDA-approved therapies to treat 22q.
Transaction Details
Under the terms of the definitive agreement, which was unanimously approved by the boards of directors of Harmony and Zynerba, Harmony will commence a tender offer to acquire all outstanding shares of Zynerba for a purchase price of $1.1059 in cash per share, or $60 million in the aggregate payable at closing of the transaction plus one non-tradeable CVR representing the right to receive potential additional payments of up to $140 million or approximately $2.5444 in additional cash per share, for a total potential consideration of up to $200 million in cash. The CVR is payable subject to certain terms and conditions upon achievement of the following milestones:
Clinical Milestones
Completion of FXS Phase 3 clinical trial: $15 million in the aggregate or approximately $0.2747 per share
Positive data readout from FXS Phase 3 clinical trial:
$30 million in the aggregate or approximately $0.5494 per share if completed on or before December 31, 2024
$20 million in the aggregate or approximately $0.3663 per share if completed on or before June 30, 2025
$10 million in the aggregate or approximately $0.1831 per share if completed after June 30, 2025
Regulatory Milestones
FDA approval in FXS: $35 million in the aggregate or approximately $0.6389 per share
FDA approval in Second Indication: $15 million in the aggregate or approximately $0.2707 per share
Net Sales Milestones
Achievement of $250 million in aggregate Net Sales: $15 million in the aggregate or approximately $0.2702 per share
Achievement of $500 million in aggregate Net Sales: $30 million in the aggregate or approximately $0.5405 per share
Each CVR is subject to the achievement of the milestone conditions described above, and there can be no assurance whether any such milestones will be achieved or when any payments will be made with respect to any CVR.
Harmony will fund the transaction from its existing cash on hand. As of June 30, 2023, Harmony had cash, cash equivalents and investment securities of $429.6 million. Zynerba's existing cash and cash equivalent balance was approximately $36.0 million as of June 30, 2023.
The transaction is expected to close by the fourth quarter of 2023, subject to customary closing conditions, including that the holders of at least a majority of the outstanding shares of Zynerba's common stock tender such shares to Harmony in connection with the tender offer. Following the successful closing of the tender offer, Harmony will acquire any shares of Zynerba it does not already own through a second-step merger at the same per share offer price as paid in the tender offer. Zynerba's board of directors unanimously recommends that Zynerba's stockholders tender their shares in the tender offer.
Advisors
For Harmony, Hogan Lovells US LLP is acting as legal counsel. For Zynerba, MTS Health Partners, L.P. is acting as financial advisor and Goodwin Procter LLP is acting as legal counsel.
Conference Call Today at 8:30 AM ET
At 8:30 AM ET Harmony will host a live webcast to review this proposed acquisition. The live and replay webcast of the call will be available on the investor relations page of our website at https://ir.harmonybiosciences.com/. To participate in the live call by phone, dial (800) 245-3047 (domestic) or +1 (203) 518-9765 (international), and reference passcode HRMY0814.
About Harmony Biosciences
At Harmony Biosciences, we specialize in developing and delivering treatments for rare neurological diseases that others often overlook. We believe that where empathy and innovation meet, a better life can begin for people living with neurological diseases. Established by Paragon Biosciences, LLC, in 2017 and headquartered in Plymouth Meeting, PA, our team of experts from a wide variety of disciplines and experiences is driven by our shared conviction that innovative science translates into therapeutic possibilities for our patients, who are at the heart of everything we do. For more information, please visit www.harmonybiosciences.com.
About Zynerba Pharmaceuticals, Inc.
Zynerba Pharmaceuticals is the leader in innovative pharmaceutically produced, synthetic transdermal cannabidiol therapies for orphan neuropsychiatric disorders. We are committed to improving the lives of patients and their families living with severe, chronic health conditions including Fragile X syndrome and 22q11.2 deletion syndrome. Learn more at www.zynerba.com.
About Fragile X Syndrome
Fragile X syndrome (FXS) is a rare genetic disorder that is the leading known cause of both inherited intellectual disability and autism spectrum disorder, affecting 1 in 3,600 to 4,000 males and 1 in 4,000 to 6,000 females. The disorder negatively affects synaptic function, plasticity and neuronal connections, and results in a spectrum of intellectual disabilities and behavioral symptoms, such as social avoidance and irritability. There are approximately 80,000 people in the U.S. and approximately 121,000 people in the European Union and UK living with FXS. There is a significant unmet medical need in patients living with FXS as there are currently no FDA approved treatments for this disorder.
FXS is caused by a mutation in FMR1, a gene which modulates a number of systems, including the endocannabinoid system, and most critically, codes for a protein called FMRP. The FMR1 mutation manifests as multiple repeats of a DNA segment, known as the CGG triplet repeat, resulting in deficiency or lack of FMRP. FMRP helps regulate the production of other proteins and plays a role in the development of synapses, which are critical for relaying nerve impulses, and in regulating synaptic plasticity. In people with full mutation of the FMR1 gene, the CGG segment is repeated more than 200 times, and in most cases causes the gene to not function. Methylation of the FMR1 gene also plays a role in determining functionality of the gene. In approximately 60% of patients with FXS, who have complete methylation of the FMR1 gene, no FMRP is produced, resulting in dysregulation of the systems modulated by FMRP.
About 22q11.2 Deletion Syndrome
22q11.2 deletion syndrome (22q) is a disorder caused by a small missing piece of the 22nd chromosome. The deletion occurs near the middle of the chromosome at a location designated q11.2. It is considered a mid-line condition, with physical symptoms including characteristic palate abnormalities, heart defects, immune dysfunction, and esophageal / GI issues, as well as debilitating neuropsychiatric and behavioral symptoms, including anxiety, social withdrawal, ADHD, cognitive impairment and autism spectrum disorder. It is estimated that 22q occurs in one in 4,000 live births, suggesting that there are approximately 80,000 people living with 22q in the U.S. and 129,000 in the European Union and the UK. Patients with 22q deletion syndrome are managed by multidisciplinary care providers, and there are currently no FDA approved treatments for this disorder.
Forward Looking Statements
This press release contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. All statements contained in this press release that do not relate to matters of historical fact should be considered forward-looking statements. These forward-looking statements, including as they relate to Harmony and Zynerba, the anticipated occurrence, manner and timing of the proposed transaction, the future development of their technologies and product candidates, including the development of and market opportunities for Zynerba's technology and product candidates, the future value (if any) of the contingent value rights, Harmony's strategy, and the anticipated synergies and benefits from the proposed transaction, are neither promises nor guarantees, but involve known and unknown risks, uncertainties and other important factors that may cause actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements. Potential risks, uncertainties and other factors to be considered include, among others, that Zynerba stockholders may not tender a sufficient number of shares in the tender offer; the length of time necessary to consummate the proposed transaction may be longer than anticipated, or it may not be consummated at all; problems may arise in successfully integrating the business and technologies of Harmony and Zynerba, and Harmony may not realize the expected benefits of the proposed transaction; the proposed transaction may involve unexpected costs; the businesses may suffer as a result of uncertainty surrounding the proposed transaction, including difficulties in maintaining relationships with third parties or retaining key employees; and even if the transaction is consummated no contingent consideration may become payable. For further discussion of these and other risks and uncertainties, see Harmony's and Zynerba's most recent Form 10-K and Form 10-Q filings with the United States Securities and Exchange Commission (the "SEC"), including under the headings "Risk Factors." You are cautioned to not place undue reliance on forward-looking statements, which speak only as of the date of this document. Except as required by law, neither Harmony nor Zynerba is under any duty to update any of the information in this document.
Additional Information about the Acquisition and Where to Find It:
In connection with the proposed acquisition, Harmony will commence a tender offer for the outstanding shares of Zynerba. The tender offer has not yet commenced. This document is for informational purposes only and is neither an offer to purchase nor a solicitation of an offer to sell shares of Zynerba, nor is it a substitute for the tender offer materials that Harmony and Xylophone Acquisition Corp ("Purchaser") will file with the SEC upon commencement of the tender offer. At the time the tender offer is commenced, Harmony and Purchaser will file tender offer materials on Schedule TO, and Zynerba will file a Solicitation/Recommendation Statement on Schedule 14D-9 with the SEC with respect to the tender offer. Holders of shares of Zynerba common stock are urged to read the tender offer materials (including an Offer to Purchase, a related Letter of Transmittal and certain other tender offer documents) and the Solicitation/Recommendation Statement when they become available (as each may be amended or supplemented from time to time) because they will contain important information that holders of shares of Zynerba common stock should consider before making any decision regarding tendering their shares. The Offer to Purchase, the related Letter of Transmittal and certain other tender offer documents, as well as the Solicitation/Recommendation Statement, will be made available to all holders of shares of Zynerba at no expense to them. The tender offer materials and the Solicitation/Recommendation Statement will be made available for free at the SEC's website at www.sec.gov. In addition, these materials will be available at no charge on the Enhanced SEC Filings section of the Investor Relations page of Zynerba's website at https://www.zynerba.com/ and by directing a request to the information agent for the tender offer, whose information will be set forth in the Offer to Purchase.
Harmony Biosciences Contacts:
Investor:
Luis Sanay, CFA
445-235-8386
lsanay@harmonybiosciences.com
Media:
Cate McCanless
202-641-6086
cmccanless@harmonybiosciences.com
Zynerba Pharmaceuticals Contact:
Peter Vozzo
ICR Westwicke
443-213-0505
Peter.Vozzo@westwicke.com
https://c212.net/c/img/favicon.png?sn=DC82854&sd=2023-08-14 View original content to download multimedia:https://www.prnewswire.com/news-releases/harmony-biosciences-to-acquire-zynerba-pharmaceuticals-inc-301899483.html
SOURCE Harmony Biosciences and Zynerba Pharmaceuticals, Inc.
Back to News Headlines
Other Financial Information
Recent News & Disclosure Filings
Recent SEC Filings
Pretty ballsy move in light of the announced and impending reverse split. They always work out so well, ya know...
It took a while... I see now they plan to reverse split this POS into oblivion.
Very good buying opportunities! Going to pass $1.60?????
ZYNE is showing a very positive technicals to continue an upward move!!!!!BUY!!!!!
Maybe not????????????
Lol.. that was Zyne's big run up for 2022..
A shame this pig won't even move except for moving down on good news. https://www.proactiveinvestors.com/companies/news/966938/zynerba-pharmaceuticals-further-boosts-patent-portfolio-for-its-zygel-cbd-gel-966938.html
Any idea why the 10% drop yesterday? I didn't see any news?
Plenty of lurkers.. not much to talk about
what happened to this message board? no one post anymore? is there a better message board for ZYNE? can someone help me please?
Lots of buying activity the past 2 days. Anyone else watching this? I'm bull long but also love this ticker for the flips
On May 14, settled the securities class action for $4,000,000. While purchasers of ZYNE who bought between March 11, 2019 and September 17, 2019, both dates inclusive (the “Class Period”) can submit a proof of claim, longer holders who bought before March 11, 2019 can seek governance reforms and a return of funds to the company from D&O policies - as well as potentially a court approved incentive award where appropriate.
To learn more, message me here or at jgrabar@grabarlaw.com
Zynerba could not meet quorum at a recent shareholder meeting. Vote people!!
zyne may just be that wild card you were
waiting for.I got in today with 1G shares,gonna ride for a easy 5 hundi.
$zyne $4.62 v -0.14 (-2.94%)
Volume: 1,196,812 @03/26/21 7:59:30 PM EDT
$zyne $4.57 ^ 0.11 (2.47%)
Volume: 1,570,084 @02/26/21 7:59:31 PM EST
Buy buy buy. $zyne $sndl & $chnc the three kings will rise again
* * $ZYNE Video Chart 02-10-2021 * *
Link to Video - click here to watch the technical chart video
$zyne $5.15 ^ 0.64 (14.19%)
Volume: 21,669,399 @02/05/21 7:59:51 PM EST
* * $ZYNE Video Chart 02-05-2021 * *
Link to Video - click here to watch the technical chart video
* * $ZYNE Video Chart 02-03-2021 * *
Link to Video - click here to watch the technical chart video
Blessings on ZYNE today.
And my God will meet all your needs according to the riches of his glory in Christ Jesus.
Philippians 4:19
$zyne $3.6 v -0.02 (-0.55%)
Volume: 2,370,826 @01/21/21 7:55:14 PM EST
$zyne $3.45 ^ 0.01 (0.29%)
Volume: 1,020,326 @12/22/20 7:30:27 PM EST
The market absofreakinlutely hates this stock.
Anyone listen to the CC this morning?
Are you suggesting a hoax? Thanks!!!
We are happy to announce the presentation of two posters at the @AmEpilepsySoc 2020 virtual meeting. You can download the posters here: https://t.co/yztO29XqkI #epilepsy $ZYNE pic.twitter.com/O0U3qoPHGt
— Zynerba Pharma (@ZynerbaPharma) December 4, 2020
Makes sense. Press Release was found under Zynerba on ETrade site entitled .............“Zynerba Pharma announces expansion of Zanitadatamab pivotal trial in HER2 amplified Biliary tract cancer trial in Asia.......”. It Didn’t make sense at all and I Couldn’t find anything about it on Zynerba website. Guess it was just and big error on ETrade’s part and a typo in the title of the press release. Thanks Ihub58
This is not Zynerba
Another company
Similar ticker, but ZYME
Good read.......no,I WAS NOT AWARE.
dhbuzz, not sure if you saw the new PR from Zynerba regarding aBiliary tract cancer study they are involved in. They recieved 10M dollars for meeting a specific milestone of treating the first patient in the study. This was a surprise to me because I was not aware of this study at all. If you know more can you expound?
lol....no sheet man..........
$zyne $4.61 ^ 0.03 (0.66%)
Volume: 370,480 @11/27/20 2:29:14 PM EST
$zyne $3.78 ? 0.0 (0.00%)
Volume: 0 @-
|
Followers
|
91
|
Posters
|
|
|
Posts (Today)
|
0
|
Posts (Total)
|
2676
|
|
Created
|
08/06/15
|
Type
|
Free
|
| Moderators | |||

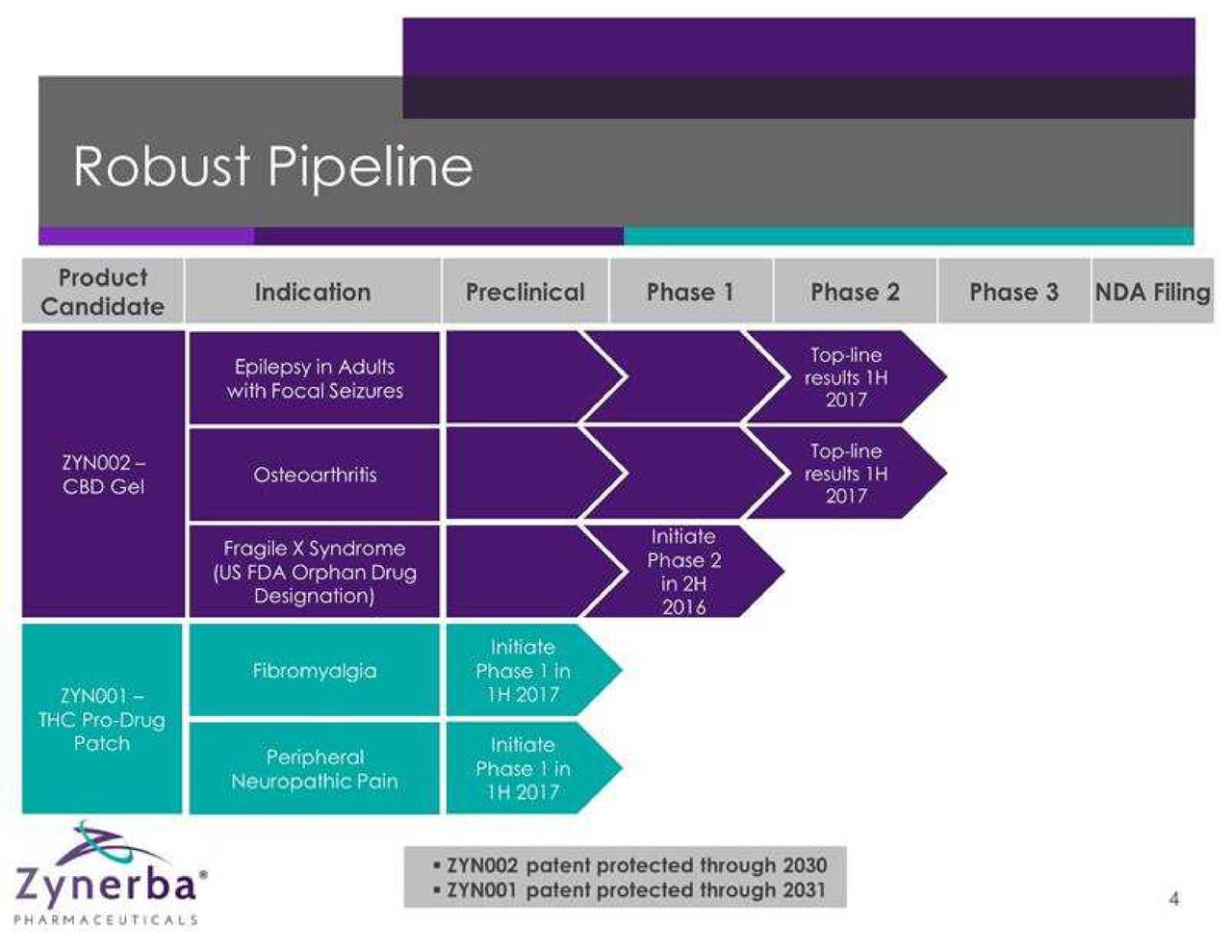
Zynerba Pharmaceuticals, Inc. is a specialty pharmaceutical company focused on developing and commercializing synthetic cannabinoid therapeutics formulated for transdermal delivery. The Company is evaluating approximately two product candidates, ZYN002 and ZYN001, in over five indications. The Company intends to study ZYN002 in patients with refractory epilepsy, osteoarthritis and Fragile X syndrome. The Company's ZYN002 is synthetic cannabidiol (CBD) formulated as a permeation-enhanced gel for transdermal delivery. ZYN002 is being developed as a clear that is designed to provide controlled drug delivery with once- or twice-daily dosing. ZYN001 is a pro-drug of tetrahydrocannabinol (THC) that enables transdermal delivery through a patch. The Company intends to test the ZYN001 patch for application to the arm, back and thigh. The Company intends to study ZYN001 in patients with fibromyalgia and peripheral neuropathic pain.
| Corporate Profile |
Zynerba (NASDAQ: ZYNE) is pioneering the development of patent-protected, next-generation synthetic cannabinoid therapeutics formulated for transdermal delivery. Its two lead product candidates in development include ZYN002 and ZYN001, which are being evaluated in five indications. ZYN002 is the first and only synthetic cannabidiol (CBD) formulated as a permeation-enhanced gel for transdermal delivery. In June 2016, the company initiated the STAR 1 Phase 2 clinical trial in refractory epilepsy patients and in August 2016, initiated the STOP Phase 2 clinical trial in patients with osteoarthritis of the knee. A Phase 2 clinical trial in patients with Fragile X syndrome will be initiated in the second half of 2016.
ZYN001, a prodrug of THC that enables transdermal delivery through the skin and circulatory system via a patch, is in preclinical development. A Phase 1 clinical trial is planned in the first half of 2017.
In August 2015, Zynerba completed an initial public offering, raising net proceeds of $42.1 million. As of June 30, 2016, cash and cash equivalents totaled $32.1 million, which is projected to fund five Phase 2 clinical trials through 2017.
| EPS (TTM) | 9/30/2016 | -2.47 |
|---|---|
| P/E Ratio | 9/30/2016 | -- |
| Market Cap | Micro Cap | 146M |
| Shares Outstanding | 9.95M |
| Float | 7.2M |
| Volume | |
| Day Range: | |
| Bid Price | |
| Ask Price | |
| Last Trade Time: |