Register for free to join our community of investors and share your ideas. You will also get access to streaming quotes, interactive charts, trades, portfolio, live options flow and more tools.
Saniona AB: The Nomination Committee of Saniona Proposes Election of Two Additional Members of the Board of Directors (GlobeNewswire)
2017-12-22 08:00
"December 22, 2017
Saniona, a leading biotech company in the field of ion channels, today announced that its Nomination Committee comprised of Søren Skjærbæk appointed by Jørgen Drejer, John Haurum appointed by Thomas Feldthus and Claus Bræstrup, Saniona's Chairman of the Board, have proposed J. Donald deBethizy and Anna Ljung as new members of the Board of Directors and that J. Donald deBethizy is elected as new Chairman of the Board of Directors."
https://www.avanza.se/placera/pressmeddelanden/2017/12/22/saniona-saniona-ab-the-nomination-committee-of-saniona-proposes-election-of-two-additional-members-of-the-board-of-directors.html
Saniona AB: Saniona Spin-Out, Scandion Oncology Raises DKK 2 Million and Prepares for a Potential Public Listing (GlobeNewswire)
2017-12-19 09:45
December 19, 2017
Saniona, a leading biotech company in the field of ion channels, today announced that its spin-out company, Scandion Oncology, has raised DKK 2 million in a private placement. Scandion intends to use the proceeds to develop its lead compound, SCO-101, for the treatment of chemotherapy resistant cancer and to mature the company for a potential public listing.
https://www.avanza.se/placera/pressmeddelanden/2017/12/19/saniona-saniona-ab-saniona-spin-out-scandion-oncology-raises-dkk-2-million-and-prepares-for-a-potential-public-listing.html
"Saniona to Present at the 10th Annual Biotech Showcase (GlobeNewswire)
2017-12-12 16:00"
"Saniona, a leading biotech company in the field of ion channels, today announced that it is scheduled to present at the 10th Annual Biotech Showcase, being held on January 8-10, 2018 at the Hilton San Francisco Union Square in San Francisco, CA.
https://www.avanza.se/placera/pressmeddelanden/2017/12/12/saniona-saniona-to-present-at-the-10th-annual-biotech-showcase.html";
Saniona Selects Preclinical Candidate in GABAA a2/a3 Program for Neuropathic Pain and Chronic Itching (GlobeNewswire) 2017-12-04 08:00
https://www.avanza.se/placera/pressmeddelanden/2017/12/04/saniona-saniona-ab-saniona-selects-preclinical-candidate-in-gabaa-a2a3-program-for-neuropathic-pain-and-chronic-itching.html
Saniona AB: Saniona publishes its interim report for the third quarter 2017 (GlobeNewswire)
2017-11-15 08:00
"Within the next two years, we will have the opportunity to initiate clinical studies in several metabolic diseases including obesity, diabetes and fatty liver/NASH. As these studies tend to be costly, we envision finding partners prior to initiating Phase 3 clinical trials. Tesomet is also potentially relevant for various eating disorders."
http://hugin.info/172499/R/2149075/824689.pdf
PRESS RELEASE
October 26, 2017
Saniona, a leading biotech company in the field of ion channels, today announces that nine out of a planned ten adult patients with Prader-Willi syndrome have completed or discontinued from the Phase 2 clinical study. Some patients showed indication of weight loss and reduced craving for food, the key efficacy endpoints. Some patients discontinued the study due to adverse effects, which might be drug related. This interim analysis will last about 2-3 months and based on the results Saniona will decide on the next steps for Tesomet in Prader-Willi syndrome.
"Prader-Willy syndrome is a complex disease with a high occurrence of concomitant co-morbidities. Many patients have major behavioural and psychiatric abnormalities at the time they reach adulthood and communication represents a challenge when performing clinical studies. Based on our observations, we see a need to un-blind the study and analyse data on individual patient's drug exposure in relation to observed efficacy and safety signals before we decide on next steps", says Jørgen Drejer, CEO of Saniona.
The actual assignment of patients to respective treatment arms is currently unknown since the placebo controlled study is still blinded. The indications of a reduction in weight and craving for food are encouraging whereas the discontinuation of five patients due adverse effects, potentially drug related, may indicate that we have used a too high dose in this study.
"We highly appreciate the dedicated and professional efforts from the primary investigators Dr. Torok in Hungary and Dr. Kolouskova in the Czech Republic and their staff to find and manage these demanding patients", says Jørgen Drejer, CEO of Saniona.
For more information, please contact
Thomas Feldthus, EVP and CFO, Saniona, Mobile: +45 2210 9957, E-mail: tf@saniona.com
https://www.avanza.se/placera/pressmeddelanden/2017/10/26/saniona-saniona-ab-saniona-decides-to-perform-interim-analysis-of-tesomet-study-in-patients-with-prader-willi-syndrome.html
Saniona's Partner Cadent Therapeutics Receives Milestone from Novartis
https://globenewswire.com/news-release/2017/10/17/1148665/0/en/Saniona-AB-Saniona-s-Partner-Cadent-Therapeutics-Receives-Milestone-from-Novartis.html
Saniona obtains research milestone from The Michael J. Fox Foundation for Parkinson's Research
https://globenewswire.com/news-release/2017/04/20/962499/0/en/Saniona-AB-Saniona-obtains-research-milestone-from-The-Michael-J-Fox-Foundation-for-Parkinson-s-Research.html
"Global childhood obesity rises 10-fold in 40 years"
"The number of obese children and teenagers across the world has increased 10-fold over the past four decades and is about to overtake the number who are underweight, according to the most extensive analysis of body weight ever undertaken."
https://www.ft.com/content/3433cfdc-adaa-11e7-aab9-abaa44b1e130?mhq5j=e5
PRESS RELEASE
2017-09-18 08:00
Saniona AB: Saniona reports encouraging start for tesofensine Phase 3 study (GlobeNewswire)
"Saniona, a leading biotech company in the field of ion channels, today announces that Saniona's partner, Medix, has recruited 150 out of the planned 372 patients in the study during the first six weeks."
https://www.avanza.se/placera/pressmeddelanden/2017/09/18/saniona-saniona-ab-saniona-reports-encouraging-start-for-tesofensine-phase-3-study.html
PRESS RELEASE
October 3, 2017
"Saniona, a leading biotech company in the field of ion channels, today announces that it has reached the third research milestone for identifying new drug candidates for the treatment of Parkinson's disease, which is supported by The Michael J. Fox Foundation for Parkinson's Research (MJFF)."
https://www.avanza.se/placera/pressmeddelanden/2017/10/03/saniona-saniona-ab-saniona-obtains-research-milestone-from-the-michael-j-fox-foundation-for-parkinsons-research.html
[t][/t]
|
Followers
|
1
|
Posters
|
|
|
Posts (Today)
|
0
|
Posts (Total)
|
10
|
|
Created
|
09/28/17
|
Type
|
Free
|
| Moderators | |||
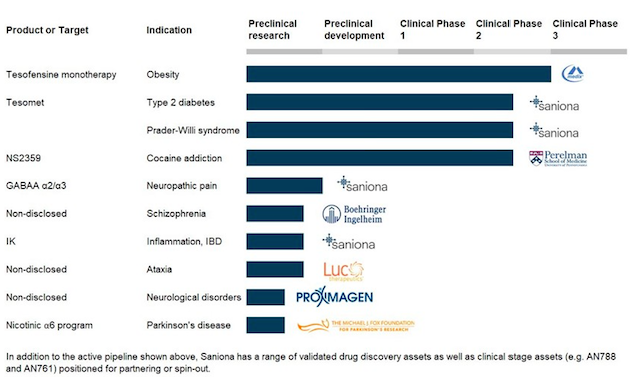
Saniona currently has clinical stage candidates for obesity, metabolic diseases and cocaine addiction and has been able to secure collaborations with top pharmaceutical companies.
Saniona has a significant portfolio of potential drug candidates at pre-clinical and clinical stage. Saniona has ongoing collaboration agreements with Boehringer Ingelheim GmbH, Proximagen Ltd., Productos Medix, S.A and Luc Therapeutics, Inc.
Tesomet and Tesofensine are the most advanced programs.
Tesofensine
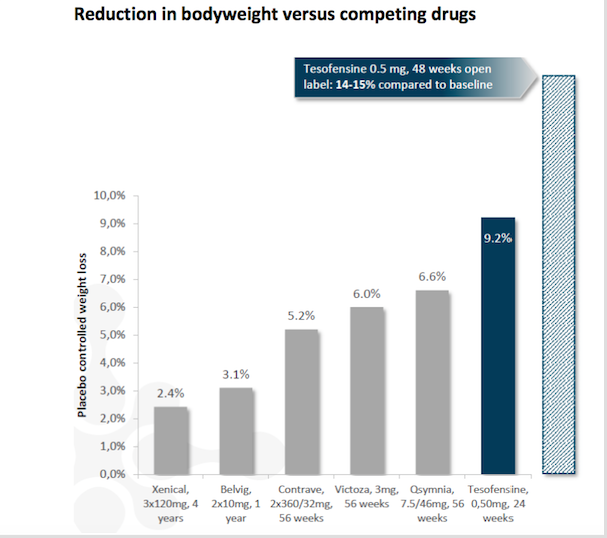
Tesofensine has been evaluated in Phase 1 and Phase 2 human clinical studies with the aim of investigating treatment potential with regards to obesity, Alzheimer’s disease and Parkinson’s disease. Tesofensine demonstrated strong weight reducing effects in Phase 2 clinical studies in obese patients. In general, tesofensine has been administered to more than 1,300 patients and is well tolerated.
"The clinical Phase 2b trial (TIPO-1), reported in The Lancet, showed levels of weight loss over a six-month period that were of high clinical relevance and highly competitive to other approaches. Patients lost an average of 12.8 kg on a 1 mg dose, 11.3 kg on a 0.5 mg dose and 6.7 kg on a 0.25 mg dose compared with a 2.2 kg loss in the placebo group"
Saniona is collaborating with Medix on developing tesofensine for obesity in Mexico and Argentina. According to Medix, the current market for prescription medicine for obesity in Mexico is about US$ 250 million. In August 2017, Medix initiated a Phase 3 study for tesofensine in obese Mexican patients. The trial is expected to be completed within two years.
'
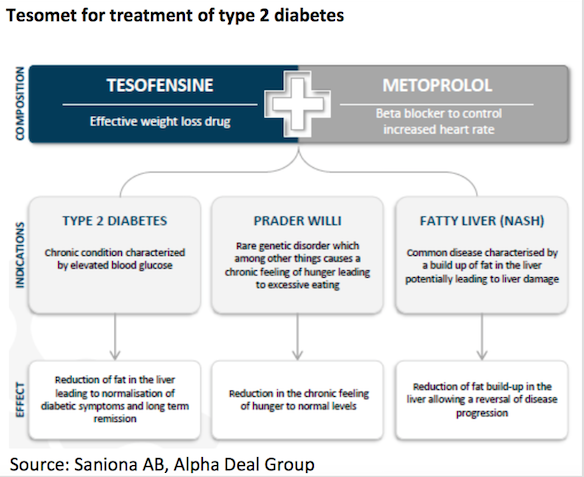
Tesomet
Tesomet is a fix-dosed combination of tesofensine and metoprolol. In 2016, Saniona performed a Phase 2a clinical trial for Tesomet in type 2 diabetes patients. Top line data from this clinical trial was presented in January 2017. The clinical trial achieved a positive outcome on the primary endpoint with a statistically significant reduction in heart rate for patients treated with Tesomet compared to placebo. Furthermore, the key secondary and exploratory endpoints regarding body weight and waist circumference also showed statistically significant reductions compared to placebo.
Tesomet has recently shown positive results in its Phase2a study for type 2 diabetes and the Company is currently conducting a Phase 2a study in patients suffering from Prader-Willi syndrome. Acc. to Datamonitor, it is expected that the type 2 diabetes market will grow from $23.3bn in 2014 to $43bn in 2023.

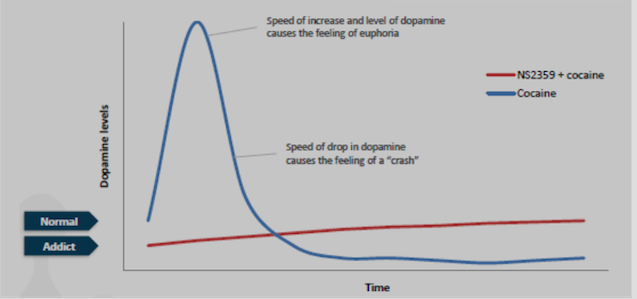
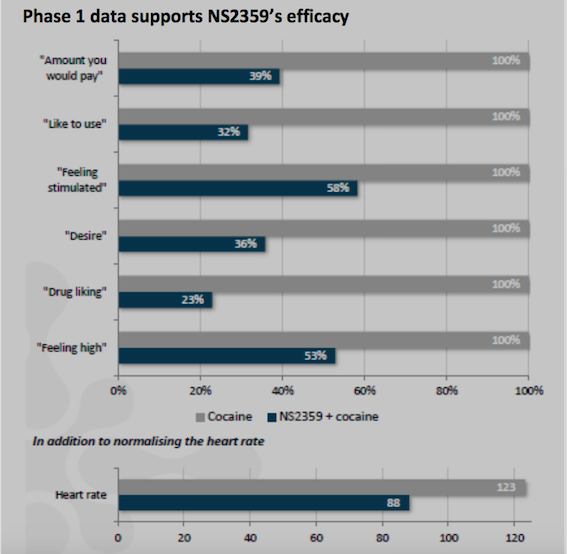
“We are very excited about the initiation of this important Phase 2a study.
TRC is world class within clinical trials on drug addiction.
The two principal investigators are
recognized leaders in addiction research. Dr. Berrettini is an
internationally-recognized expert in the
genetics of addiction and Dr. Kampman is a highly respected top
researcher in conducting clinical studies of cocaine addiction"
| Volume | |
| Day Range: | |
| Bid Price | |
| Ask Price | |
| Last Trade Time: |
