Register for free to join our community of investors and share your ideas. You will also get access to streaming quotes, interactive charts, trades, portfolio, live options flow and more tools.
OTLC
Oncotelic Therapeutics Inc (QB)
0.023385
-0.000515 (-2.15%)
Volume: 5,220
Day Range: 0.02295 - 0.023385
Last Trade Time: 1:31:48 PM EDT
OTLC
Oncotelic Therapeutics Inc (QB)
0.0205
-0.00035 (-1.68%)
Volume: 320,370
Day Range: 0.0205 - 0.022
Last Trade Time: 4:06:18 PM EDT
OTLC
Oncotelic Therapeutics Inc (QB)
0.0345
0.004 (13.11%)
Volume: 718,034
Day Range: 0.031 - 0.035
Last Trade Time: 4:02:37 PM EDT
OTLC
Oncotelic Therapeutics Inc (QB)
0.033
-0.002 (-5.71%)
Volume: 161,792
Day Range: 0.033 - 0.0355
Last Trade Time: 4:01:43 PM EDT
OTLC
Oncotelic Therapeutics Inc (QB)
0.042
-0.002 (-4.55%)
Volume: 265,396
Day Range: 0.042 - 0.0525
Last Trade Time: 3:21:30 PM EDT
OTLC
Oncotelic Therapeutics Inc (QB)
0.051
0.01 (24.39%)
Volume: 42,028
Day Range: 0.0425 - 0.052
Last Trade Time: 3:41:20 PM EDT
Oncotelic Announces Release of Pet2DAO Tokens (PDAO): https://www.benzinga.com/pressreleases/23/04/g31629187/oncotelic-announces-release-of-pet2dao-tokens-pdao
OTLC
Oncotelic Therapeutics Inc (QB)
0.048
-0.00705 (-12.81%)
Volume: 73,296
Day Range: 0.0451 - 0.055
Last Trade Time: 3:55:10 PM EST
OTLC
Oncotelic Therapeutics Inc (QB)
0.048
-0.00705 (-12.81%)
Volume: 73,296
Day Range: 0.0451 - 0.055
Last Trade Time: 3:55:10 PM EST
OTLC
Oncotelic Therapeutics Inc (QB)
0.055
0.001 (1.85%)
Volume: 65,321
Day Range: 0.04815 - 0.055
Last Trade Time: 2:35:44 PM EST
OTLC
Oncotelic Therapeutics Inc (QB)
0.0465
-0.0015 (-3.13%)
Volume: 55,357
Day Range: 0.046 - 0.0495
Last Trade Time: 3:34:32 PM EST
yes /\ Oncotelic Therapeutics Inc.
The balance sheet and cap structure shows that this management team did a toxic financing which means the stock will go down. That is 100% sure to occur because toxic financings benefit the financier because the stock is given by the company to the financier at a discount to market.
The financier sells the stock, even at a lower price than the market and they guarantee themselves a profit.
That is how and why you see 0001 a share price for some stocks.
Another reason this stock will go down is because I once spoke to the CFO - he was is stupid he has no right being an officer of a public company.
I am guessing that he is an accountant and the CEO hired him to be a figurehead.
Anytime a CFO is stupid you get stupid decisions and then the stock goes down.
Charts don't matter.
The balance sheet and cap structure shows that this management team did a toxic financing which means the stock will go down. That is 100% sure to occur because toxic financings benefit the financier because the stock is given by the company to the financier at a discount to market.
The financier sells the stock, even at a lower price than the market and they guarantee themselves a profit.
That is how and why you see 0001 a share price for some stocks.
Another reason this stock will go down is because I once spoke to the CFO - he was is stupid he has no right being an officer of a public company.
I am guessing that he is an accountant and the CEO hired him to be a figurehead.
Anytime a CFO is stupid you get stupid decisions and then the stock goes down.
#248
$MATN & $GILD?????,6;41a.m. 07-07-2020 .M. Mateon Therapeutics Appts. mick 07/28/2020 10:25:22 PM
OTLC
Oncotelic Therapeutics Inc (QB)
0.06
0.00355 (6.29%)
Volume: 301,282
Day Range: 0.0551 - 0.06
Bid: 0.0484
Ask: 0.0761
Last Trade Time: 3:53:06 PM EDT
Total Trades: 27
OTLC Detailed Quote
Management is inept
Once I spoke to the CFO and he clearly does not know anything about being a CFO of a public company.
The CEO on the other hand does not understand the importance of letting investors know about his company. He keeps raising capital from investors at such bad terms the only people that will ever make money are his bankers and the people that continue to short the stock.
Some people have no right to raise capital because although they are not a scam, there is no chance of their stock increasing because management is inept or they don't understand the markets.
OTLC
Oncotelic Therapeutics Inc (QB)
0.08077
0.00027 (0.34%)
Volume: 563,673
Day Range: 0.08 - 0.082
Bid: 0.0681
Ask: 0.09
Last Trade Time: 3:44:59 PM EDT
Total Trades: 29
OTLC Detailed Quote
OTLC
Oncotelic Therapeutics Inc (QB)
0.10
-0.003 (-2.91%)
Volume: 292,973
Day Range: 0.10 - 0.103
Bid: 0.098
Ask: 0.10
Last Trade Time: 4:07:43 PM EDT
Total Trades: 37
OTLC Detailed Quote
Surprised at the lack of interest
Looks like we should expect success in the Mesothelioma trial around the six month time frame. I am sure that it will take many would be short players off guard, and make them regret their little games.
Oncotelic Announces Pipeline Presentation and IPO Update of Sapu Therapeutics at the 2022 BIO International Convention: https://finance.yahoo.com/news/oncotelic-announces-pipeline-presentation-ipo-120000848.html
Oncotelic Therapeuti (OTLC)
0.21 ? 0.009 (4.48%)
Volume: 64,267 @06/03/22 3:53:07 PM EDT
Bid Ask Day's Range
0.175 0.224 0.196 - 0.214
OTLC Detailed Quote
ONCOTELIC PROVIDES Q1 2022 FINANCIAL RESULTS COMPARED TO Q1 2021, PRODUCT DEVELOPMENT INITIATIVES AND CORPORATE UPDATE: https://www.globenewswire.com/news-release/2022/05/23/2448615/0/en/ONCOTELIC-PROVIDES-Q1-2022-FINANCIAL-RESULTS-COMPARED-TO-Q1-2021-PRODUCT-DEVELOPMENT-INITIATIVES-AND-CORPORATE-UPDATE.html
Oncotelic Appoints Fatih Uckun, M.D., Ph.D., as Chief Medical Officer: https://oncotelic.irpass.com/Oncotelic-Appoints-Fatih-Uckun--M-D---Ph-D---as-Chief-Medical-Officer-5-3-2022
Oncotelic Appoints Seymour Fein M.D. as Chief Regulatory Officer: https://oncotelic.irpass.com/Oncotelic-Appoints-Seymour-Fein-M-D--as-Chief-Regulatory-Officer-5-4-2022
Oncotelic Therapeuti (OTLC)
0.216 ? -0.0112 (-4.93%)
Volume: 60,460 @04/29/22 3:54:24 PM EDT
Bid Ask Day's Range
0.211 0.2279 0.2123 - 0.2279
OTLC Detailed Quote
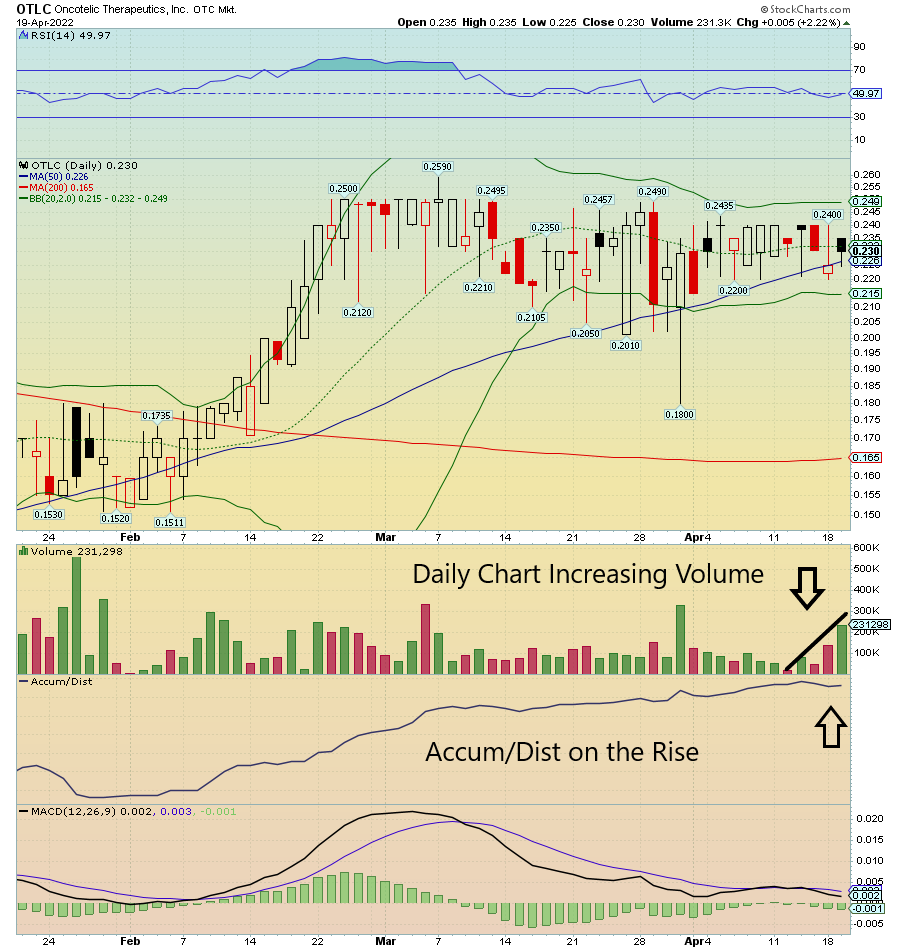
$OTLC on watch due to Daily Chart increasing Volume and now crossing the 50 SMA. Accum/Dist on the rise as well.
Recently provided Year 2021 Financial Results:
https://www.globenewswire.com/news-release/2022/04/18/2423605/0/en/ONCOTELIC-PROVIDES-YE-2021-FINANCIAL-RESULTS-COMPARED-TO-YE-2020-PRODUCT-DEVELOPMET-INITIATIVES-AND-CORPORATE-UPDATE.html
$OTLC ONCOTELIC PROVIDES YE 2021 FINANCIAL RESULTS COMPARED TO YE 2020, PRODUCT DEVELOPMET INITIATIVES AND CORPORATE UPDATE
AGOURA HILLS, Calif., April 18, 2022 (GLOBE NEWSWIRE) -- Oncotelic Therapeutics, Inc. (“Oncotelic”, “We” or the “Company”) (OTCQB:OTLC) today announced financial results for the full year ended December 31, 2021 (“FY 2021”) as compared to the full year ended December 31, 2020 (“FY 2020”), an update on its product and therapeutic development initiatives and other corporate updates. The financial results were based on the 2021 Annual Report on Form 10-K filed with the Securities and Exchange Commission on April 15, 2022.
https://www.otcmarkets.com/stock/OTLC/news/ONCOTELIC-PROVIDES-YE-2021-FINANCIAL-RESULTS-COMPARED-TO-YE-2020-PRODUCT-DEVELOPMET-INITIATIVES-AND-CORPORATE-UPDATE?id=352529
This Little Known Cancer Play has been Crushing the Market (ADGI, OTLC, DVAX, PACB, SAVA, NVAX, IMGN, BNTX)
https://www.wallstreetpr.com/this-little-known-cancer-play-has-been-crushing-the-market-adgi-otlc-dvax-pacb-sava-nvax-imgn-bntx-46481
This Little Known Cancer Play has been Crushing the Market (ADGI, OTLC, DVAX, PACB, SAVA, NVAX, IMGN, BNTX)
https://www.wallstreetpr.com/this-little-known-cancer-play-has-been-crushing-the-market-adgi-otlc-dvax-pacb-sava-nvax-imgn-bntx-46481
$OTLC Company Profile and Technical Chart Analysis
$OTLC Letter to Oncotelic Shareholders on the Recently Completed Joint Venture Transaction with Dragon Overseas Capital Limited
AGOURA HILLS, Calif., April 07, 2022 (GLOBE NEWSWIRE) -- Oncotelic Therapeutics, Inc. (“Oncotelic” or the “Company”) (OTCQB:OTLC), issued the following shareholder letter today.
Dear Fellow Shareholders,
We would like to thank you for being part of our mission at Oncotelic which is to develop treatments for cancer patients that have limited options. Our efforts include pursuing broad applications of our technology as well as the treatment of cancer types that are given orphan drug designation (“ODD”) meaning there are a limited number of patients that have a high unmet medical need. We have spent considerable time and effort to unify our collective assets toward the achievement of this goal. This effort included receiving a Rare Pediatric Disease (“RPD”) designation for pediatric cancer which give us access to the RPD voucher on top of the regulatory exclusivity reserved for ODD while we continue strategic planning for broader applications of our technologies. Taken together, these efforts give us the potential to produce a strong return on investment for our shareholders
Earlier this week, we announced together with our partner Dragon Overseas Capital Limited (“Dragon Overseas”), that we have formed a joint venture (the “JV”) for the discovery, development and commercialization TGF-ß therapeutics against all pharmaceutical indications. The JV intends to initially focus on the further development and commercialization of OT-101, including for DIPG as well as pancreatic cancers and glioblastoma, which are devasting diseases with few treatment options. In connection with this, the Company has granted the JV global, exclusive, perpetual, royalty-free license of intellectual property rights, including and in respect of OT-101 (the “License”).
Our TGF-beta drug candidate- OT-101 has completed seven clinical trials including one phase 2 trial in COVID and two phase 2 trials in brain cancer and against pancreatic cancer. We received FDA designation for pediatric designation related to a rare form of pediatric brain cancer known as diffuse intrinsic pontine glioma (“DIPG”). There are approximately 200-300 new cases of DIPG every year in the United States. DIPG most often occurs in children ages 5-10 years old. Treatment options are limited with surgery being contraindicated. Most children do not survive more than 2 years after diagnosis. Currently, the main treatment for DIPG is radiation therapy. Although radiation temporarily improves symptoms in most patients, it is not a cure. Palliative care or quality of life services help patients and families manage pain and other symptoms, promote quality of life, and making difficult decisions including treatment choices and end of life care. At Oncotelic, we are dedicated to the treatment of DIPG and welcome our shareholders on this endeavor.
The anti-viral activity of OT-101, in an in vitro antiviral testing performed by an independent laboratory, OT-101 has an 50% effective concentration (EC50) of 7.6 µg/mL and is not toxic at the highest dose of 1000 µg/mL giving a safety index (SI) value of >130, which is considered highly active and on par or superior to Remdesivir. Unlike Remdesivir, OT-101 targets not only the virus replication but also the virus induced pneumonia and fibrosis. Our Phase 2 trial was completed for OT-101 in South America. This was a randomized, double-blind, placebo-controlled Phase 2 study intended to evaluate the safety and efficacy of OT-101 in adult patients hospitalized with positive SARS-CoV-2 and pneumonia. As reported on November 23, 2021, the top line data is positive for safety and efficacy.
This transaction allows us to unburden the Company of the high cost of drug development, which the JV will be responsible for, while the Company will participate in its upside through appreciation in the value of its shares in the JV and up to $50MM on the sale of the RPD voucher following marketing approval of OT-101 for DIPG. Dragon Overseas has agreed to invest cash and other assets with a value of approximately $27.6MM for 55% ownership of the JV; and Oncotelic has granted the License to the JV for 45% ownership in the JV.
The fuel needed to drive OT-101 development is robust financing- and the JV provides that. This combination of strong financial backing and strong asset creates a solid foundation for the coming IPO. We believe that the JV IPO will be as successful as other recent IPOs in the TGF-ß space. We believe that there is a substantial upside for the Company due to this JV especially if we are able to have a successful IPO. Your attention is drawn to Litchfield’s analyst, Theodore R. O’Neill’s recent coverage on the Company. (https://www.oncotelic.com/wp-content/uploads/2022/03/OTLC-JV-update.pdf).
In the coming months the team will be transitioning into the JV to ensure continuity of operational excellence. Look for future announcements on clinical trials undertaken by the JV in collaboration with pharmaceutical houses and Key Opinion Leaders “KOLs”. Dr. Anthony Maida, our current CCO, will drive the JV’s clinical development of OT-101 in combination with checkpoint inhibitors including pembrolizumab. Multiple phase 2 clinical trials are intended to be launched with our collaborators. Dr. Maida believes that this Joint Venture provides the resources and financial support for Oncotelic allowing to execute its clinical strategy in multiple solid tumor indications coupled with a robust and thorough bioinformatics assessment leading to upstream targeting, improved clinical responses and insights into agnostic applications.
We appreciate your continued support in our development of cures for orphan and rare diseases. By combining innovative drugs and robust financing we look to transform healthcare one rare disease at a time.
Sincerely yours,
Vuong Trieu and the Oncotelic team
About Oncotelic
Oncotelic (f/k/a Mateon Therapeutics, Inc.), was formed in the State of New York in 1988 as OXiGENE, Inc., was reincorporated in the State of Delaware in 1992, and changed its name to Mateon Therapeutics, Inc. in 2016, and Oncotelic Therapeutics, Inc. in November 2020. Oncotelic is seeking to leverage its deep expertise in oncology drug development to improve treatment outcomes and survival of cancer patients with a special emphasis on rare pediatric cancers. Oncotelic has rare pediatric designation for DIPG (OT-101), melanoma (CA4P), and AML (OXi 4503). Oncotelic also acquired PointR Data Inc. in November 2019.
Additionally, Oncotelic acquired AL-101, during the 4th quarter of 2021, for the intranasal delivery of apomorphine. We intend to develop AL-101 for the treatment of Parkinson Disease (“PD”). Over 60,000 new patients are being diagnosed with PD in the United States and currently there are over 1 million patients in the US and expected to increase to over 1.2 million by 2030. In addition, approximately 10 million suffer from this disease globally. https://www.parkinson.org/Understanding-Parkinsons/Statistics. AL-101 is also being developed for Erectile Dysfunction (“ED”). ED is the most prevalent male sexual disorder globally. The percentages of men affected by ED are as follows: 14.3-70% of men aged ≥60 years, 6.7-48% of men aged ≥70 years, and 38% of men aged ≥80 years (Geerkens MJM et al. (2019). Eur Urol Focus. pii: S2405-4569(19)30079-3). However, with the increasing administration of PDE5 inhibitors in clinical practice, it was found that approximately 30-35% of ED patients are treatment failures (McMahon CN et al. (2006). BMJ, 332: 589-92). AL-101 is designed to target treatment failure ED patients who do not respond to PDE5 inhibitors. Through similar mechanism of action, AL-101 is being developed for Female Sexual Dysfunction (“FSD”). Female sexual dysfunction is a prevalent problem, afflicting approximately 40% of women and there are few treatment options. FSD is more typical as women age and is a progressive and widespread condition. (Allahdadi, KJ et al. (2009) Cardiovascular & hematological agents in medicinal chemistry, 7(4), 260–269). There is no available drug for the treatment of FSD. In June 2019, the U.S. Food and Drug Administration approved Vyleesi (bremelanotide) to treat acquired, generalized hypoactive sexual desire disorder (“HSDD”) in premenopausal women. This is the only available drug treatment. Vyleesi has essentially replaced the only other drug for HSDD – however, it has a long list of drug-drug interactions, including commonly used antidepressants, such as fluoxetine and sertraline. In addition, it has a black box warning regarding its use with alcohol, a combination that has been associated with hypotension and syncopal episodes. Therefore, there is an urgent need for effective therapy against FSD and HSDD.
For more information, please visit www.oncotelic.com
https://www.globenewswire.com/news-release/2022/04/07/2418633/0/en/Letter-to-Oncotelic-Shareholders-on-the-Recently-Completed-Joint-Venture-Transaction-with-Dragon-Overseas-Capital-Limited.html
OTLC a bargain here, The JV will be IPO at significant valuation. The consequences are: 1) OTLC shares will increase in value proportionally as we now own 45% of the JV 2) Those who invest in OTLC will be getting in the IPO on the ground level as OTLC is now proxy to the IPO
Oncotelic; Announcing Successful Joint Venture and Diving into Benefits for Shareholders:
Oncotelic presentation at MedInvest Pharmaceutical & Biotechnology Investor Conference:
|
Followers
|
14
|
Posters
|
|
|
Posts (Today)
|
0
|
Posts (Total)
|
956
|
|
Created
|
08/23/16
|
Type
|
Free
|
| Moderators | |||




Oncotelic is actively seeking collaborations with corporations, academic institutions, and patient groups to advance our programs.
We have a history of successful partnerships and welcome new collaboration and licensing opportunities.
Interested parties may contact:
Chulho Park PhD, Chief Technology Officer
Oncotelic Inc.
29397 Agoura Rd., Suite 107
Agoura Hills, CA 91301
bd@oncotelic.com
(650) 635-7000
Through our history of collaborative research, Mateon has supported clinical research projects in the United Kingdom (UK) led by
Cancer Research UK (CRUK) along with additional academic partners.
Currently, we are supporting the PAZOFOS study (NCT02055690), which is being led by The Christie Hospital NHS Foundation Trust
and coordinated by the Manchester Academic Health Science Centre,
Trials Coordination Unit, or MAHSC-CTU, with additional support from The University of Manchester, the Royal Marsden NHS Foundation Trust,
and Mount Vernon Cancer Centre (part of the East and North Hertfordshire NHS Trust).
This Phase 1b/2 study is evaluating the combination of CA4P (fosbretabulin) with the tyrosine kinase inhibitor pazopanib (Votrient®) versus pazopanib
alone for the treatment of advanced recurrent ovarian cancer.
We are providing CA4P for use in this trial.
Continuing in our history of collaborative research, Mateon is providing investigational drug to the Markey Cancer Center for their study Study MCC-2016-08 (NCT03014297).
The study is designed as a single center, open label, phase 1 clinical trial for patients with grade 1-3 gastroenteropancreatic neuroendocrine tumors.
In the first part of the study, up to 15 patients will be treated with everolimus in combination with two different dosing regimens of
CA4P to establish appropriate CA4P dosing levels and evaluate the safety of the drug combination.
The second part of the study is designed to enroll 15 additional patients for assessment of additional safety and efficacy data.
Patients enrolled in MCC-2016-088 will be treated with CA4P and everolimus for 12 weeks.
We are committed to leveraging our intellectual property and the product development expertise of our highly experienced management team to enable
vascular targeted therapy to realize its true potential and to bring much needed new therapies for orphan oncology indications to cancer patients worldwide.
We have a portfolio of preclinical and clinical stage candidates focused on areas of significant unmet need that we believe can bring significant value to our shareholders.
Our common stock is traded on the OTCQB Market under the symbol “MATN”.
We are headquartered in Agoura Hills, California
https://www.oncotelic.com/pointr-data/
EdgePoint AI was established in order to advance the company’s revolutionary cluster-computer platform for AI that processes machine learning
models at a fraction of the power and budget of mainstream computing in pharmaceutical manufacturing.
The company’s technology solution “TrustPoint” provides an AI computing platform for pharmaceutical and healthcare verticals
including blockchain support for manufacturing where data integrity and security are of utmost importance.
EdgePoint is composed of a team of executives with pharmaceutical drug development, GMP manufacturing and deep AI knowledge.
The team includes Steven King who was the former CEO of Peregrine Pharmaceuticals, Inc. (NASDAQ) and its wholly-owned
contract biomanufacturing subsidiary Avid Bioservices, Inc., for over 15 years, during which time the company advanced its
lead compound through Phase 3 development, while growing revenues to over $55 million;
Seasoned executive, Saran Saund who has been founder, CEO and GM at several startups and public companies for which he returned
significant value to shareholders in his startups and chief technology officer; Burcak Beser who has been founder and
CTO of several successful private and public companies with over 144 patents during his career; and Lisa Stepp with broad experience in
executive leadership across geographies and in all aspects of drug development and program management.
The core technology platform for EdgePoint AI came from PointR -which was acquired by Mateon in Nov 2019. Examples of PointR capabilities are shown below.

http://oncotelic.com/wp-content/uploads/LosAngelesBusinessJournal2018-05-21.pdf
Health Care: Drug Developer Adds Digital Monitor
TWO YEAR CHART: NASDAQ
| PER IHUB MGMT |
02-07-2021
DISCLAIMER: ONLY FOR MICK
https://investorshub.advfn.com/boards/profilea.aspx?user=1012
*The Board Monitor and herewithin , are not licensed brokers and assume NO responsibility for actions,
investments,decisions, or messages posted on this forum.
CONTENT ON THIS FORUM SHOULD NOT BE CONSIDERED ADVISORY NOR SOLICITATION
AUTHORS MAY HAVE BUYS OR SELLS WITH THE COMPANIES MENTIONED IN TRADING POSTERS SHOULD DUE DILIGENT BUYING OR SELLING.
ALL POSTING SHOULD BE CONSIDERED FOR INFORMATION ONLY. WE DO NOT RECOMMEND ANYONE BUY OR SELL ANY SECURITIES POSTED HEREWITHIN.
ANY trade entered into risks the possibility of losing the funds invested.
• There are no guarantees when buying or selling any security.Any
| Volume | |
| Day Range: | |
| Bid Price | |
| Ask Price | |
| Last Trade Time: |
