Saturday, July 15, 2017 4:27:09 PM
7-14-17/CEO Steve King: “On the R&D side, we are pleased to report that the company continues to make progress with its PS-targeting Exosome Diagnostic Technology that is designed to detect & monitor cancer. Our scientists have successfully optimized the assay and we are currently preparing to generate addl. data, testing the diagnostic with an expanded set of human samples. Such data will be important to partnering discussions and we’ll keep you posted on progress. http://tinyurl.com/yb4wulvu
- - - - - - - - - -
**6-22-17: Nature/BJoC article, Dr. Alan Schroit/UTSW etal: "Detection of PS+ Exosomes for the Diagnosis of Early-Stage Breast & Pancreatic [Using Betabody KL15C]" http://tinyurl.com/ycrx9672 <= This article has not been PR’d or mentioned as yet by PPHM, to my knowledge.
**2-9-17/PR: PS+ Exosomes Proof-of-Concept Data (N=44, Ovarian, Dr. Alan Schroit/UTSW etal) pub. 1-22-17 in OncoTarget http://tinyurl.com/jhv57ua
From the 7-14-17 PR: http://tinyurl.com/yb4wulvu
PS EXOSOME TECHNOLOGY HIGHLIGHTS:
The company continues to make progress with its PS exosome diagnostic technology that is designed to detect and monitor cancer. The assay has been successfully optimized and we are currently preparing to generate addl. data by testing human samples. Such data will be important to partnering discussions.
4-30-17 10-K pub. 7-14-17 pg.6: http://tinyurl.com/ycxu4l5n
Exosome Technology
We licensed from UTSWMC in July 2016, proprietary Exosome-Based Cancer Diagnostic Technology, with the goal of developing an optimized test for further clinical testing in collaboration with a potential partner. The platform is based on the diagnostic potential of tumor derived exosomes, which are small vesicles from tumor cells that are released into the blood as tumors grow. Tumor derived exosomes have PS on their surface as a potentially detectable marker. It is believed that even small tumors begin to release PS-positive exosomes and thus the ability to detect these exosomes in the blood may be an indicator of the presence of a tumor. In the study published by Oncotarget [1-22-17: http://tinyurl.com/yb4wulvu ], plasma samples from 34 patients with ovarian tumors and 10 healthy subjects were analyzed for the presence of PS-expressing exosomes in a blinded test. Results demonstrated that those patients with malignant ovarian cancer displayed significantly higher blood PS exosome levels than those with benign tumors (median 0.237 vs. -0.027, p=0.0001) and the malignant and benign groups displayed significantly higher blood PS exosome levels than the healthy subjects (median 0.237 vs -0.158, p < 0.0001 and -0.027 vs -0.158, p=0.0002, respectively).
.
.
= = = = = = = = = = = = = = = = = = = = = = = = = = =
PPHM’s Exosomes Diag. Platform (incl. 6-22-2017 NATURE/BJC article)
...Known POC Data Summary (see below):
1-22-17/OncoTarget: Ovarian – senior author Alan Schroit (UTSW/PPHM SAB)
6-22-17/Nature(BJC): Breast & Pancreatic – senior author Alan Schroit (UTSW/PPHM SAB) *Using Betabody KL15C*
PPHM’s PS+ EXOSOME-BASED CANCER DETECTION & MONITORING TECHNOLOGY ("Liquid Biopsy") – Reverse Chron. History
...Excellent Exosome (aka microparticles, microvesicles) info: http://www.exosome-rna.com
6-22-17: Nature (British Jrnl of Cancer): ”Detection of Phosphatidylserine-Positive Exosomes for the Diagnosis of Early-Stage Malignancies [Using Betabody KL15C]”
Raghava Sharma, Xianming Huang, Rolf A Brekken (PPHM SAB), Alan J Schroit
….Dr. Schroit: PPHM SAB, UTSW profile: http://tinyurl.com/yaqnvcvq ; pubs: http://tinyurl.com/lqbd8kt
https://www.nature.com/bjc/journal/vaop/ncurrent/pdf/bjc2017183a.pdf
ABSTRACT
Background: There has been increasing interest in the detection of tumor exosomes in blood for cancer diagnostics. Most studies have focused on miRNA and protein signatures that are surrogate markers for specific tumor types. Because tumor cells and tumor-derived exosomes display phosphatidylserine (PS) in their outer membrane leaflet, we developed a highly sensitive ELISA-based system that detects picogram amounts of exosomal phospholipid in plasma as a cancer biomarker.
Methods: This report describes the development of a highly specific and sensitive ELISA for the capture of PS-expressing tumor exosomes in the blood of tumor-bearing mice. To monitor the relationship between tumor burden & tumor exosome plasma concentrations, plasma from one transplantable breast cancer model (MDA-MB-231) and 3 genetic mouse models (MMTV-PyMT; breast and KIC and KPC; pancreatic) were screened for captured exosomal phospholipid.
Results: We show that quantitative assessment of PS-expressing tumor exosomes detected very early-stage malignancies before clinical evidence of disease in all 4 model systems. tumor exosome levels showed significant increases by day 7 after tumor implantation in the MDA-MB-231 model while palpable tumors appeared only after day 27. For the MMTV-PyMT and KIC models, tumor exosome levels increased significantly by day 49 (P<=0.0002) and day 21 (P<=0.001) while tumors developed only after days 60 & 40, respectively. For the KPC model, a significant increase in blood exosome levels was detected by day 70 (P=0.023) when only preinvasive lesions are microscopically detectable.
Conclusions: These data indicate that blood PS exosome levels is a specific indicator of cancer and suggest that blood PS is a biomarker for early-stage malignancies.
- - - - -
Addl. article info (Fig.1) extracted by Bamboozler762 6-23-17 iHub #300359 http://investorshub.advfn.com/boards/read_msg.aspx?message_id=132436726
Fig1: Schematic diagram of betabody, KL15C
- - - - - - - - - -
Note: BETABODIES (Clipped/Nicked B2GPI - ex: KL15, “2nd-gen. PS-Targeting”) - bind to PS directly, are smaller in size (100 vs. 250KDa) and have a longer serum half-life (~5days) than natural antibodies (Bavi=~1day) – see http://tinyurl.com/khopa3d …2/17/15: UTSW/PPHM’s BetaBodies patent#8,956,616 Awarded(Granted) http://tinyurl.com/p75uyfu

Fig.1: Schematic diagram of betabody, KL15C
Domains 1 & 5 of the plasma PS-binding protein, B2GP1, were genetically fused to the C-terminus of the CH3 domains of the Fc fragment of human IgG1. A Gly4Ser linker was inserted between the CH3 domains and domains 1 and 5 of B2GP1. The recombinant betabody was expressed as a dimer.
- - - - - - - - - -
...Glimpse of beg. of 7pg. 6-22-17 BJC article: http://www.readcube.com/articles/10.1038/bjc.2017.183

2-23-17/StockNewsUnion.com: “What Next For Peregrine Pharmaceuticals After Exosomes Proof of Concept?”
Peregrine has said that its sight is on developing the technology into an optimized diagnostic platform for clinical testing of cancer. To reach that goal, Peregrine said it is seeking strategic partners to help it develop & commercialize the platform. Those partners would be expected to inject money into the project for an equity stake in PPHM or a cut of revenue from the sale of the platform. There are several large pharmaceutical companies developing their own ovarian cancer diagnostic systems are in need of such technology, so PPHM could pitch to them to join its exosomes-based diagnostic efforts.
POTENTIAL PARTNERS:
* AstraZeneca plc (NYSE:AZN https://www.astrazeneca.com - http://finance.yahoo.com/quote/AZN MktCap=$72B)
* Tesaro Inc. (NASDAQ:TSRO http://www.tesarobio.com - http://finance.yahoo.com/quote/TSRO MktCap=$9.5B)
* Clovis Oncology Inc. (NASDAQ:CLVS http://clovisoncology.com - http://finance.yahoo.com/quote/CLVS MktCap=$2.6B)
...are some of the major pharma brands that could be a fit for PPHM. The pharma giants are at various stages in the development of their ovarian cancer assets.”
http://stocknewsunion.com/what-next-for-peregrine-pharmaceuticals-nasdaqpphm-after-proof-of-concept/2043/
2-9-17/PR: ”Recent Publication Highlights Proof-of-Concept Data Supporting the Diagnostic Potential of Phosphatidylserine-Positive Exosomes in Ovarian Cancer”
http://ir.peregrineinc.com/releasedetail.cfm?ReleaseID=1011223
TUSTIN, Feb. 9, 2017: Peregrine Pharmaceuticals, Inc. (NASDAQ:PPHM/PPHMP), a biopharmaceutical company committed to improving patient lives by manufacturing high quality products for biotechnology and pharmaceutical companies and advancing its proprietary R&D pipeline, today announced the publication of positive proof-of-concept data for a novel exosome-based cancer detection platform. Results of the study, conducted at University of Texas (UT) Southwestern Medical Center, showed researchers were able to distinguish between healthy subjects and patients with ovarian tumors based on the levels of exosomes containing phosphatidylserine (PS) found in their plasma. Furthermore, analysis of the PS-positive exosome levels allowed researchers to distinguish between malignant and benign tumors. These data were recently published online by the peer-reviewed journal, Oncotarget, in a paper titled, "Detection of Phosphatidylserine-Positive Exosomes as a Diagnostic Marker for Ovarian Malignancies: A Proof-Of-Concept Study."
Peregrine is currently advancing the proprietary exosome-based cancer diagnostic technology, licensed from UT Southwestern Medical Center in July 2016 [7-14-16: http://tinyurl.com/zszd4fj ], with the goal of developing an optimized test for further clinical testing. As part of these efforts, the company is in the process of seeking a strategic partner for collaboration on developing and commercializing the technology. The platform is based on the diagnostic potential of tumor exosomes, which are small vesicles from tumor cells that are released into the blood as tumors grow. Tumor derived exosomes have PS on their surface as a detectable marker. It is believed that even small tumors begin to release PS-positive exosomes and thus the ability to detect these exosomes in the blood may be an indicator of the presence of a tumor.
In the study published by Oncotarget [1-22-17 FULL article: http://tinyurl.com/hlc68tq ], plasma samples from 34 patients with ovarian tumors and 10 healthy subjects were analyzed for the presence of PS-expressing exosomes in a blinded test. Results demonstrated that those patients with malignant ovarian cancer displayed significantly higher blood PS exosome levels than those with benign tumors (median 0.237 vs. -0.027, p=0.0001) and the malignant and benign groups displayed significantly higher blood PS exosome levels than the healthy subjects (median 0.237 vs -0.158, p < 0.0001 and -0.027 vs -0.158, p=0.0002, respectively).
"These initial proof-of-concept results are encouraging as they appear to support the underlying concept that the measurement of PS-positive exosome levels in blood could be a simple way to detect and monitor cancer. While the work is still early, we think these data serve as an important first step in highlighting the diagnostic potential of this platform," said Steven W. King, President and CEO of Peregrine. "This type of diagnostic technology is particularly important in an area such as ovarian cancer, in which screening options are limited and the ability to detect the disease at an early stage is inadequate. We look forward to continuing to explore the potential of the technology platform in ovarian as well as other types of cancer."
"There is a significant and growing interest in the healthcare industry around the ability to detect cancer and monitor its progression with more readily accessible blood tests. With this area being one of the fastest growing segments of the oncology diagnostics market, we believe that our exosome-based technology represents a significant product development and licensing opportunity," stated Stephen Worsley, VP of Business Development at Peregrine. "Based on the fact that PS is a marker associated with a broad range of cancer types, we believe our platform has potential applications in several solid tumors beyond ovarian cancer. With that in mind, we look forward to aligning with a partner to help explore the potential of this promising technology."
- - - - - - - - - - - - -
1-22-17/OncoTarget Article: PS-Exosomes: PPHM SAB’r Dr. Alan Schroit: Proof-of-Concept Datasupports the “high diagnostic power” of PS-Positive Exosomes in Ovarian Malignancies. ...Basically, Dr. Schroit (cell membrane guru) & his UTSW team used a variant of fully-human Bavi (1N11) to detect the PS on the exosomes…
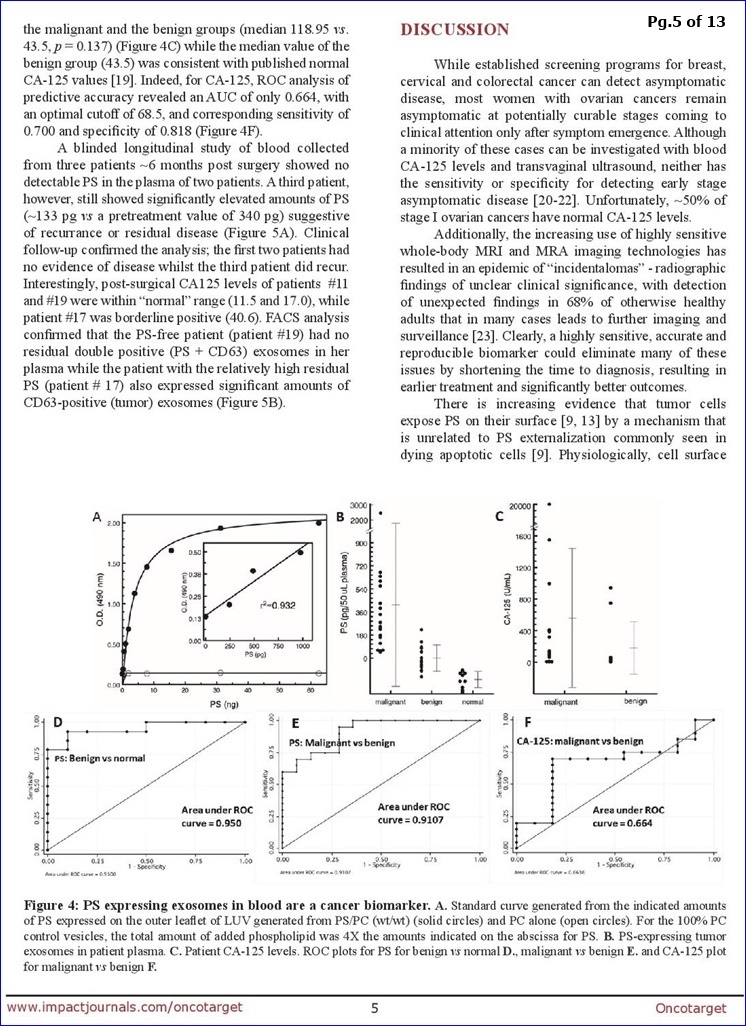
FROM PG.8: “The data summarized in Fig. 4 [N=44] show that quantification of PS-exosomes in blood distinguishes, with 100% accuracy, healthy tumor-free individuals from patients with ovarian malignancies.”
1-22-17 Oncotarget:
“Detection of Phosphatidylserine-Positive Exosomes as a Diagnostic Marker for Ovarian Malignancies: A Proof of Concept Study”
Lea J 1, Sharma R 2, Yang F 2, Zhu H 1, Ward ES 3,4, Alan J. Schroit (PPHM SAB, UTSW Profile: http://tinyurl.com/yaqnvcvq ) 1,3
1 Harold Simmons Comprehensive CC, UTSW-MC/Dallas
2 Hamon Center for Therapeutic Oncology Res., UTSW-MC/Dallas
3 Dept of Immunology, UTSW-MC/Dallas
4 Dept of Molecular & Cellular Medicine, Texas A&M Univ. Health Science Ctr
https://www.ncbi.nlm.nih.gov/pubmed/28122335 Rec.1-4-17, Acc.1-11-17, Pub.1-22-17
ABSTRACT:
There are no suitable screening modalities for ovarian carcinomas (OC) and repeated imaging and CA-125 levels are often needed to triage equivocal ovarian masses. Definitive diagnosis of malignancy, however, can only be established by histologic confirmation. Thus, the ability to detect OC at early stages is low, and most cases are diagnosed as advanced disease. Since tumor cells expose phosphatidylserine (PS) on their plasma membrane, we predicted that tumors might secrete PS-positive exosomes into the bloodstream that could be a surrogate biomarker for cancer. To address this, we developed a highly stringent ELISA that detects picogram quantities of PS in patient plasma. Blinded plasma from 34 suspect ovarian cancer patients and 10 healthy subjects were analyzed for the presence of PS-expressing vesicles. The nonparametric Wilcoxon rank sum test showed the malignant group had significantly higher PS values than the benign group (median 0.237 vs. -0.027, p=0.0001) and the malignant & benign groups had significantly higher PS values than the healthy group (median 0.237 vs -0.158, p<0.0001 and -0.027 vs -0.158, p=0.0002, respectively). ROC analysis of the predictive accuracy of PS-expressing exosomes/vesicles in predicting malignant against normal, benign against normal & malignant against benign revealed AUCs of 1.0, 0.95 and 0.911, respectively. This study provides proof-of-concept data that supports the high diagnostic power of PS detection in the blood of women with suspect ovarian malignancies.
FULL ARTICLE (13pgs):
HTML: http://www.impactjournals.com/oncotarget/index.php?journal=oncotarget&page=article&op=view&path%5B%5D=14795&path%5B%5D=47251
PDF: http://www.impactjournals.com/oncotarget/index.php?journal=oncotarget&page=article&op=download&path%5B%5D=14795&path%5B%5D=47248
EXCERPTS FROM 1-22-17 ONCOTARGET ARTICLE:
**From DISCUSSION (pg. 8): “The data summarized in Fig. 4 show that quantification of PS-exosomes in blood distinguishes, with 100% accuracy, healthy tumor-free individuals from patients with ovarian malignancies… In summary, this study provides proof-of-concept data that supports the high diagnostic power of PS-expressing tumor exosome detection in blood from women with suspect ovarian malignancies. Ultimately, these studies could lead to earlier stage diagnosis, substantial cost savings, reduced patient exposure to radiation and invasive procedures, and improved clinical outcomes. The assay might also find utility in patients with radiographic abnormalities, even before clinical detection. Indeed, an accurate biomarker predicting the likelihood of malignancy would be extremely beneficial to such a population since they often face long periods of anxiety and uncertainty inherent to a “wait & watch” approach. Finally, if PS-exosome diagnostics are confirmed in a large study to be an accurate and reproducible biomarker of ovarian malignancies, the assay could be applied to the early detection of other visceral malignancies."
**From METHODS (pg.9): “Expression of an engineered tetravalent antibody for PS-detection Monoclonal 1N11 is a human IgG1^ that binds PS through the PS-specific plasma protein B2GP1. A tetravalent variant of 1N11 (1N11-T), with 4 binding sites per molecule was designed to generate a high avidity PS binding agent (Fig.1).”
……...[NOTE: 1N11 is Fully-Human Bavituximab (aka PGN635=AT004), B2GPI-dep. Binding]
**From FINANCIAL SUPPORT (pg.11): “Supported by Cancer Prevention & Res. Inst. of TX (CPRIT) Grant #RP110441, and a Simmons CC Support Grant 5P30 CA142543.”
**EDITORIAL NOTE (pg.11): “This paper has been accepted based in part on peer-review conducted by another journal and the authors’ response & revisions as well as expedited peer-review in Oncotarget."
.
PDF EXCERPTS 1-22-17/OncoTarget/A.Schroit/PPHM-Exosomes (5 pgs of the 13):




NOTE/2-10-17: Exosomes-RNA.com posted the news of the 2-9-17 PPHM Exosomes POC PR...
http://www.exosome-rna.com/proof-of-concept-data-for-a-novel-exosome-based-cancer-detection-platform-published/

10-13-16/ASM REPORT BY ATTENDEE COPPER888: “...I think there is a shift on how the company execs and BOD view the business. As mentioned by multiple posters, SK said that they are still looking to hit a "homerun" with Bavi. But I think that they are now doing that in a framework of risk avoidance, and profitability as their primary goals. With every initiative mentioned, SK would talk about partnering in the next sentence. Exosome testing with a partner; potential of exploring the utility of Beta Bodies - would "advance aggressively with a partner"; If Sunrise data warrants a small study to confirm, they would "partner the next step", etc. I think that for good or bad...the new company directive is the march toward profitability. He also said that the company is worth multiples of its current market cap and that they want to delay the RS as much as they can. "I am focused on getting the Share price over a dollar" He mentioned that there may be many events between now and April that may get us there...” http://tinyurl.com/jx7ouay
9-8-16/CC J.Hutchins PREPARED: “1st, an an update on our PS Exosome Program. As we announced in July, we executed a licensing agreement with UT Southwestern Medical Center for a novel exosome technology designed to detect PS-positive exosomes in a patient blood sample. It is our belief that this technology can provide clinicians with detection & monitoring information regarding the presence and the prevalence of cancer. Preliminary indep. studies have demonstrated that the levels of PS-positive exosomes present in the blood of cancer patients are higher than levels found in the blood of healthy volunteers. Furthermore, study findings also suggest that there is a correlation between the level of PS positive exosomes detected in the blood of cancer patients and the severity and extent of their disease burden. Given our in-house expertise in PS targeting, we believe that we are uniquely qualified to advance this technology. We believe there are significant opportunities to use this technology as both a complementary tool in bavituximab's ongoing development as well as more broadly as the basis for a novel cancer detection & monitoring test kit that will be the focus of partnering efforts. It is our goal to develop, optimize and validate a functional screening assay capable of detecting PS positive exosomes in a blood sample and to initiate partnering discussions for commercialization of the pgm in 2017. We are on track to achieve this goal and we look forward to providing further updates.” http://tinyurl.com/jydtkoy
9-8-16-PR/FY17Q1: “Peregrine in-licensed a novel exosome technology from UTSW that has potential for cancer detection and monitoring applications. This technology aligns directly with the company's expertise, its proprietary PS-targeting platform and the bavituximab dev. program. As such, there are opportunities to use this technology as both a complementary tool in bavituximab's ongoing development, as well as more broadly as the basis for novel cancer detection & monitoring tests that can be the focus of partnering efforts.” http://tinyurl.com/jydtkoy
7-14-16/PR: Peregrine Licenses Exosome-based technology from UTSW (Inventors: Alan Schroit/Philip Thorpe) http://tinyurl.com/zszd4fj
...“relates to assays that are able to detect small amts of PS+ Exosomes in a patient's blood sample as a way to detect cancer at a very early stage of development.”
VP JEFF HUTCHINS: “We are excited to enter into this licensing agreement with our long-term collaborators at UTSW. This technology offers a promising product development opportunity and aligns directly with the company's expertise with our proprietary PS-targeting platform and our longstanding CDMO capabilities around the development, qualification, and validation of in vitro analytical assays. As such, there are significant opportunities to use this technology as both a complementary tool in bavituximab's ongoing development, as well as more broadly as the basis for novel cancer detection & monitoring tests that can be the focus of partnering efforts. It is important to note that this dev. program will require minimal capital investment and has the potential to create significant value over the next 18mos., including potential partnering opportunities. As a result, we feel that today's licensing deal provides yet another important driver in our ongoing efforts to achieve profitability."
7-14-16/CC S.King PREPARED: “...In addition to bavituximab program which continues to have tremendous potential value, the company announced earlier today that it has licensed-in a Novel PS Exosome Technology, with the potential to detect & monitor cancer at an early stage through a simple blood test [ 7-14-16: http://tinyurl.com/zszd4fj ]. I recognize that some of you might think this theme is contrary to controlling spending as we move toward profitability, but in fact we believe that given our already existing knowledge base in Targeting PS and our already available infrastructure for developing & validating tests, that so a very modest capital investment we can quickly reach proof-of-concept with a goal of partnering this technology, which could then bring in addl. revenue, with another potential upside of the technology being that it can possibly be useful in the continued dev. of bavituximab. Jeff will talk more about this during his prepared remarks. Given the strategy of R&D targeted toward early partnering, will allow the company to continue its R&D activities with significant upside coming from partnering as we move to our profitability.” http://tinyurl.com/h8eqtg5
7-14-16/CC J.Hutchins PREPARED: “I'm very happy to be able to discuss a number of exciting developments in our preclinical group. First, as Steve discussed, we have executed the licensing agreement with UT Southwestern Medical Center, for novel exosome technology [ 7-14-16: http://tinyurl.com/zszd4fj ]. While many of you are familiar with exosomes, I’ll provide a brief overview for those who are not. Exosomes are cell-secreted vesicles, or mini-cells if you will, that are present in nearly all bodily fluids, including blood. Likewise, tumor-derived exosomes represent small pieces of tumor cells that are released into the blood as tumors grow. As well, these tumor-derived exosomes have Phosphatidylserine or PS on their surface as a marker and can also contain DNA, RNA and proteins as markers of malignant disease. It is believed that even small tumors begin to release PS-positive exosomes, and thus the ability to detect these exosomes in the blood may be an indicator of presence or progression of a tumor. The licensed technology is designed to detect & monitor PS-positive exosomes in a patient's blood sample, providing clinicians with detection & monitoring information regarding the presence & prevalence of cancer. These exosomes have PS flipped to the outside of the surface and demonstrate immunosuppressive activity, just as we find with tumor cells. Preliminary studies have demonstrated that the levels of PS-positive exosomes present in the blood of cancer patients are higher than levels found in the blood of healthy volunteers. Furthermore, study findings also suggest that there is a correlation between the level of PS-positive exosomes that are detected in the blood of cancer patients and the severity or extent of their disease burden. Given our in-house expertise in PS-targeting, we believe that we are uniquely qualified to advance this technology. As Steve stated, there are significant opportunities to use this technology as both a complimentary tool in bavituximab's ongoing development, which Joe will address later, as well as more broadly as the basis for a novel cancer detection & monitoring test kit that will be the focus of our partnering efforts. It is our goal to develop, optimize, and validate a functional detection & monitoring assay capable of detecting PS-positive exosomes from a simple blood sample, and, given the company's extensive experience in developing assays of this type, we do not anticipate the need for added personnel or any specialized equipment for this project. Once we have successfully validated this assay, we plan to establish proof-of-concept through an efficient preclinical & clinical testing program. We have no intention of conducting further development work beyond the proof-of-concept stage. Rather, we expect to initiate partnering discussions for commercialization of this program in 2017. We're very excited to begin this work on this new program and we'll have more details to offer in the coming months.”
...SUMMARY: “And lastly, we've in-licensed a new exosome technology for a minimal cost that leverages our existing in-house expertise and provides us with another opportunity for us to create value to product development. Together, we believe the strategy will provide success, as it will allow us to focus the majority of our resources on achieving our primary corporate goal, future sustainable profitability within 24mos. At same time, we will focus our R&D efforts on small early stage trials and development of the exosome technology in an effort to attract partners. We believe this strategy will allow us to build near-term revenues through Avid, while maintaining the potential for significant addl. value creation associated with our R&D efforts.” http://tinyurl.com/h8eqtg5
7-14-16/CC J.Shan PREPARED: “I'd first like to comment on our new exosome program. One of the most exciting aspects of this technology is the potential synergy that it offers with our bavituximab clinical dev. program. Through our ongoing work with bavituximab, we have gained significant understanding of PS-mediated immuno-suppression in cancer. The availability of the PS specific biomarker, which can be implemented in our planned future bavituximab clinical trials, aligns nicely with our refocused bavituximab dev. strategy aimed at generating the most meaningful data possible from small, early stage clinical trials to support partnering efforts. We are very anxious to bring this new technology to Peregrine and we look forward to the value it brings to our bavituximab program.” http://tinyurl.com/h8eqtg5
7-14-16/CC Q&A/Pantginis – SK: “(On the Exosome pgm), …it fits right in; we’re not having to hire addl. people, we’re not having to bring in addl. equipment. This really fits in with everything we are doing on both studying PS, as well as on the assay development side of the business. We think it’s actually going to be complementary. There are other technologies out there looking at exosomes; they're are all taking a very different approach to what we’re doing and we actually think they could be very complementary to each other. We also see a need, even as interest in exosomes begins to pick up, to actually utilize this in conjunction with other things that are in development.” http://tinyurl.com/h8eqtg5
7-14-16/CC Q&A/T.Yip – SK: “(On the Exosome pgm), the next step will be to validate that through patient samples. The beauty of this is that while you do need IRB approval of course, you’re not running really clinical trials, so this can be done in conjunction with either our ongoing trials or partners' trials, or there are many other sources of just receiving these types of blood samples. This gives us the ability to very quickly go through and test hundreds or thousands of patient samples as part of the validation process. At that point, we can zero in on what are the potential applications of the technology, outside of what we might do with our own PS Targeting programs – what would be the potential utility of this for patients. Our goal is not to become a diagnostics company, but to put this in the hands of a good organization that's already established in the diagnostics area and then have them finish up the commercialization and expansion of the utility of the actual assay itself. Our benefit at that point would become, hopefully, some residual royalties, milestones and what have you, which feeds back into our revenue goals of becoming profitable. So, we thought that this is a very attractive technology that just fits right in with what we’re doing and requires almost no addl. resources whatsoever…
Q2:/TY: ”when should we expect to see more preclin. data on this front?”… SK: “Our goal is, probably towards for the end of this year, to be in a position to have data that we can present. That will come in a lot of different formats, so it will be in conjunction with other ongoing studies that maybe taking place already to be standalone just on the diagnostic itself. So, you'll be hearing a lot about this, and one of the reasons when to get this news out there is because what you think sooner than later we will build a talk about this technology.” http://tinyurl.com/h8eqtg5
6-30-16: UTSW WEBSITE - “AVAILABLE TECHNOLOGIES” (#2932):

“A Blood-based Diagnostic Test for the Early Detection of Cancer” (Alan Schroit, S.Ward, A.Gazdar)
ALL: http://www.utsouthwestern.edu/about-us/administrative-offices/technology-development/industry/available-technologies.html
SCHROIT/EXOSOMES #2932: http://www.utsouthwestern.edu/edumedia/edufiles/about_us/admin_offices/technology_development/available_technologies/utsd-2932.pdf
This invention is a highly sensitive, blood-based assay system for the diagnosis of cancer. Tumors secret large quantities of cell membrane-derived exosomes into the blood stream of cancer patients. While all exosomes, whether from tumor cells or normal cells, share membrane epitopes similar to those found on the parent cell membrane, only tumor cell-derived exosomes express the membrane phospholipid, phosphatidylserine (PS), in the outer leaflet of the particle membrane. Based on data clearly showing that PS exposure is a unique signature of tumor-derived exosomes, the investigators developed a discovery platform for the screening & detection of tumor-derived exosomal PS signatures in patient blood. This novel tool provides:
1) A diagnostic marker that could dramatically improve the early detection of cancer.
2) Predicts whether a tumor is benign or malignant.
3) Could provide a predictive marker for response to therapy. Importantly, early detection of indolent neoplastic disease could significantly improve patient survival.
Currently, there are very few blood tests for cancer. These include alpha-fetoprotein for hepatocellular cancer, CA125 for ovarian cancer, and prostate specific antigen for prostate cancer. Although, high levels of these proteins might indicate the presence of neoplastic disease, none are diagnostic. Because our system detects a shared universal marker of neoplastic disease, it provides a viable method for detecting cancer of any type, in any organ.
6-9-16/WORLD-PATENT: Pub# WO/2016/201064, Filed 6-9-16, pub. 12-15-16 (A.Schroit, S.Ward, A.Gazdar)
Title: “Diagnostic Test For Early Stage Cancer”
URL (patentscope.wipo.int): http://tinyurl.com/gqvwkga
ABSTRACT:
“Disclosed are methods of identifying tumor-derived exosomes as an early cancer diagnostic, as well as for staging, assessing progression, and assessing therapy of cancer.”
Inventors: Alan J. Schroit [UTSW, PPHM SAB]; Adi Gazdar [UTSW]; E. Sally Ward [Texas A&M]
Applicant: Board of Regents, The Univ. of Texas System, Peregrine Pharmaceuticals, Inc.
EXCERPT from SUMMARY:
“Detecting may comprise contacting the sample of step (b) with an exosome binding agent that may be the same or other phosphatidylserine binding agent than the one used in step (b), or a non-phosphatidylserine binding agent. Given that only PS-positive tumor exosomes are captured and available at this stage (after step b), the detecting agent may be an agent that binds to all exosomes regardless of their source… In general, normal tumor-free individuals have undetectable levels of PS-exosomes in an unconcentrated sample. In contrast, patients with tumors will typically exhibit values above 100 pg/50 \xh of sample in an unconcentrated sample. Thus, values greater than 50 pg/50 ui, plasma (or serum) are indicative of a malignancy. The method may further comprise obtaining said sample from the subject prior to step (a). The method may further comprise diagnosing the subject from which the sample was obtained as having cancer. The method may further comprise staging the cancer or classifying the cancer type in the subject from which the sample was obtained. The method may further comprise performing steps (a)-(c) a 2nd time, and comparing the results from the 1st & 2nd times, thereby assessing cancer progression or regression. The method may further comprise performing steps (a)-(c) a 2nd time, wherein the subject has received a cancer therapy after the 1st performance of steps (a)-(c) and before the 2nd performance of steps (a)-(c), and comparing the results from the 1st & 2nd times, thereby assessing the efficacy of cancer therapy. The method may further comprise treating the subject with a cancer therapy… [0096] In addition to PS, exosomes exhibit a number of unique & non-unique markers. Some of the exosome-specific markers include biomarkers that can help distinguish the nature of the type of cancer from which the exosome originated. Some exemplary exosome biomarkers can be found above in Table 1. Global exosome biomarkers that can be detected with appropriately labeled agents include cd9, cd63, cd81, ALIX, HSP70, TSG101, duramycin, and heparin.”

- - - - - - - - -
Here is the corresponding U.S. PATENT APPLIC: Pub# 20170146542, Filed 6-9-16, pub. 5-25-17 (A.Schroit, S.Ward, A.Gazdar)
USPTO.gov: http://tinyurl.com/y7clom9w “DIAGNOSTIC TEST FOR EARLY STAGE CANCER”
- - - - - - - - - -
NOTE: 2-21-17: Exosomes-RNA.com posts the Exosomes PPHM/Schroit World-Patent App today.
http://www.exosome-rna.com/new-patent-application-published-diagnostic-test-for-early-stage-cancer/

2-27-15/U.S.PATENT: App#20150241431, filed 2-27-15 (Schroit/Thorpe)
Title: “Methods & Compositions for Isolating Exosomes”
http://tinyurl.com/Schroit-Thorpe20150241431
ABSTRACT:
“Disclosed are surprising new methods & compositions for isolating extracellular microvesicles such as exosomes, particularly disease-related and phosphatidylserine (PS)-positive extracellular microvesicles as exemplified by tumor- and viral-derived exosomes. The methods of the invention are rapid, efficient, cost-effective and, importantly, are suitable for use with large volumes of biological fluids and produce antigenically intact extracellular microvesicles & exosomes. The methods & compositions are based on the surprising use of acetate buffers to isolate large quantities of extracellular microvesicles, particularly tumor-derived exosomes, from solution, without damaging their morphological or functional properties or antigenicity.”
Inventors: Alan J. Schroit; Philip E. Thorpe; Shelley Fussey
Applicant: Board of Regents, The Univ. of Texas System, Peregrine Pharmaceuticals, Inc.
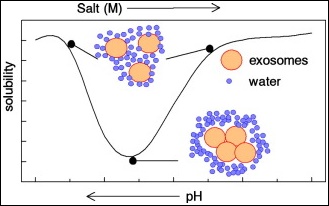
May2014/Article: J Immunol Methods, Thorpe/Schroit/etal, “A Novel ‘Salting-Out’ Procedure for the Isolation of Tumor-Derived Exosomes”
https://www.ncbi.nlm.nih.gov/pubmed/24735771
ABSTRACT:
“The last decade has seen an exponential growth in the number of exosome-related publications. Although many of these studies have used exosomes from biological fluids (blood, and ascites or pleural effusions) the vast majority employed vesicles isolated from large volumes of tissue culture supernatants. While several techniques are available for their isolation, all require a significant reduction in volume to obtain sufficient concentrations for study. One approach is to concentrate the medium before proceeding with their isolation, however, these procedures are very time consuming and require specialized laboratory equipment. Here we provide a new & effective method for the isolation of tumor-derived exosomes based on "charge neutralization" with acetate. We show that titration of tissue culture supernatants with 0.1M acetate to pH4.75 results in immediate precipitation of virtually all the exosomes. The precipitated exosomes can be washed to remove residual media and are readily "resolubilized" upon resuspension in acetate-free buffer at neutral pH. This simple cost effective method significantly increases the yield of exosomes from an unlimited quantity of culture supernatants. Exosomes isolated by this technique are indistinguishable from exosomes recovered by direct ultracentrifugation.”
Recent CDMO News
- Avid Bioservices Reports Financial Results for Third Quarter Ended January 31, 2024 • GlobeNewswire Inc. • 04/24/2024 09:25:33 PM
- Avid Bioservices Announces Receipt of Deficiency Notice from Nasdaq Regarding Late Form 10-Q • GlobeNewswire Inc. • 03/20/2024 11:00:10 AM
- Form 8-K - Current report • Edgar (US Regulatory) • 03/07/2024 11:30:11 AM
- Avid Bioservices Announces Pricing of Private Placement of Convertible Notes • GlobeNewswire Inc. • 03/07/2024 04:58:48 AM
- Avid Bioservices Announces Proposed Private Placement of Convertible Notes • GlobeNewswire Inc. • 03/06/2024 09:32:07 PM
- Avid Bioservices Announces Certain Preliminary Financial Results for Third Quarter Ended January 31, 2024 • GlobeNewswire Inc. • 03/06/2024 09:31:28 PM
- Form 8-K - Current report • Edgar (US Regulatory) • 03/06/2024 09:30:18 PM
- Form SC 13G/A - Statement of acquisition of beneficial ownership by individuals: [Amend] • Edgar (US Regulatory) • 01/26/2024 09:57:52 PM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 01/13/2024 12:34:35 AM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 01/12/2024 12:39:18 AM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 01/12/2024 12:38:30 AM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 01/12/2024 12:37:38 AM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 01/12/2024 12:36:27 AM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 01/12/2024 12:35:47 AM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 01/11/2024 12:56:02 AM
- Form SC 13G/A - Statement of acquisition of beneficial ownership by individuals: [Amend] • Edgar (US Regulatory) • 01/08/2024 09:32:36 PM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 01/04/2024 12:56:18 AM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 01/04/2024 12:55:07 AM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 01/04/2024 12:53:58 AM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 01/04/2024 12:51:57 AM
- Form SC 13G - Statement of acquisition of beneficial ownership by individuals • Edgar (US Regulatory) • 12/19/2023 09:05:52 PM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 12/19/2023 12:34:08 AM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 12/19/2023 12:33:03 AM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 12/19/2023 12:32:11 AM
FEATURED Cannabix's Breath Logix Alcohol Device Delivers Positive Impact to Private Monitoring Agency in Montana, USA • Apr 25, 2024 8:52 AM
Bantec Reports an Over 50 Percent Increase in Sales and Profits in Q1 2024 from Q1 2023 • BANT • Apr 25, 2024 10:00 AM
Kona Gold Beverages, Inc. Announces Name Change to NuVibe, Inc. and Initiation of Ticker Symbol Application Process • KGKG • Apr 25, 2024 8:30 AM
Axis Technologies Group and Carbonis Forge Ahead with New Digital Carbon Credit Technology • AXTG • Apr 24, 2024 3:00 AM
North Bay Resources Announces Successful Equipment Test at Bishop Gold Mill, Inyo County, California • NBRI • Apr 23, 2024 9:41 AM
Epazz, Inc.: CryObo, Inc. solar Bitcoin operations will issue tokens • EPAZ • Apr 23, 2024 9:20 AM










