Friday, March 10, 2017 4:35:34 PM
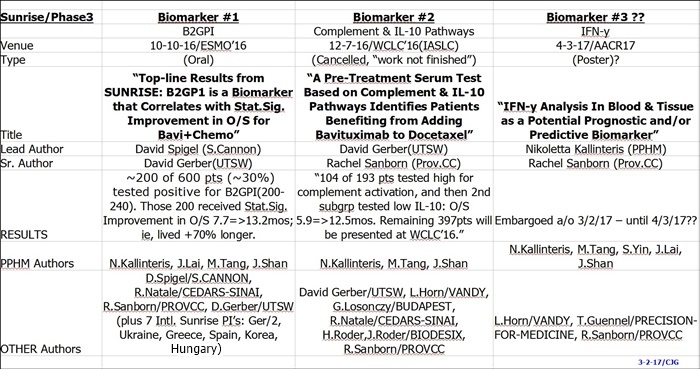
A 3rd Sunrise Biomarker (IFN-y) was revealed on 3-1-17 when the AACR’17 Abstracts were released. It will be presented on 4-3-17: #CT159/25 (Session: Phase II/III Clinical Trials in Progress). IMO(stress IMO), this 3rd Biomarker is the superior and go-forward one for Peregrine (ie, “associated with improved outcomes for future bavituximab-containing trials”), since the latest one released and the only one to be presented at AACR’17. Summary of the 3 known Ph3/Sunrise Biomarker Data Presentations:
#1 10-10-16/ESMO’16: “B2GPI Biomarker(30%pts) StatSig OS 7.7=>13.2mos.”
David R. Spigel: LEAD AUTHOR: CSO/Dir. Lung Cancer Pgm/Sarah Cannon Res.
David E. Gerber: SENIOR AUTHOR: UTSW/Dallas (Sunrise PI)
CO-AUTHORS: R.Natale/CEDARS-SINAI, R.Sanborn/PROVCC, PPHM’s N.Kallinteris, J.Lai, M.Tang, J.Shan, and 7 Intl. Sunrise PI’s: Ger/2, Ukraine, Greece, Spain, Korea, Hungry.
RESULTS:
~200 of 600 pts (~30%) tested positive for B2GPI(200-240). Those 200 received Stat.Sig. Improvement in O/S 7.7=>13.2mos; ie, lived +70% longer.
MORE DETAILS: http://tinyurl.com/hp73njt
#2 12-7-16/WCLC’16(IASLC): “Complement & IL-10 Pathways Id Pts Benefiting from Bavi+Doce”
PRESENTATION CANCELLED – IR said,”Data analysis not completed in time.”
David E. Gerber: LEAD AUTHOR: UTSW/Dallas (Sunrise PI)
Rachael Sanborn: SENIOR AUTHOR: Dir./Thoracic-Oncology, Providence CC/Portland
CO-AUTHORS: L.Horn/VANDY, G.Losonczy/BUDAPEST, R.Natale/CEDARS-SINAI, H.Roder, J.Roder/BIODESIX, PPHM’s N.Kallinteris, M.Tang, J.Shan.
RESULTS:
104 of 193 pts tested high for complement activation, and from that, a 2nd subgrp isolated that tested low IL-10: O/S 5.9=>12.5mos. Remaining 397pts will be presented at WCLC’16.
* ”The Complement System is an enzyme cascade that is a collection of blood & cell surface proteins to help the abilities of antibodies to clear pathogens from an organism.”
* ”IL-10 is an anti-inflammatory TH2 cytokine that has a critical role in limiting the immune response to pathogens to prevent host damage.”
MORE DETAILS: http://tinyurl.com/z8cq8vx
. . . .
12-12-16 CEO/S.King: “We are actively evaluating addl. potential biomarkers and we hope to identify a profile for patients who will receive therapeutic benefit from treatment with bavituximab.” VP/JOE SHAN: ”Numerous addl. biomarkers are currently being evaluated.” http://tinyurl.com/hhn4gga
#3 - REVEALED BY AACR’17 ABSTRACT REL. 3-1-17 – SUNRISE BIOMARKER #3:
4-3-17/1pm #CT159/25 (Session: Phase II/III Clinical Trials in Progress)
“IFN-y Analysis In Blood & Tissue as a Potential Prognostic and/or Predictive Biomarker” <=this abstract is embargoed by AACR’17.
Nikoletta Kallinteris (PPHM): LEAD AUTHOR: Peregrine
Rachael Sanborn: SENIOR AUTHOR: Dir./Thoracic-Oncology, Providence
CO-AUTHORS: L.Horn/VANDY, T.Guennel/PRECISION-FOR-MEDICINE, PPHM’s N.Kallinteris, M.Tang, S.Yin, J.Lai, J.Shan.
* “IFNy, or type II interferon gamma, is a cytokine that is critical for innate & adaptive immunity against viral, some bacterial & protozoal infections.”
MORE DETAILS: http://tinyurl.com/zdsbds8

= = = = = = = =BIOMARKER #3 AND PPHM/NCCN JOHNS-HOPKINS TRIAL TIE-IN (IMO):
Dr. Ranee Mehra’s (Johns-Hopkins/SidneyKimmelCC) work with Biomarker IFN-y seems to dovetail into PPHM’s newly revealed Sunrise Biomarker #3 to be presented 4-3-17 at AACR’17: #CT159/25, “IFN-y Analysis In Blood & Tissue as a Potential Prognostic and/or Predictive Biomarker” ( http://tinyurl.com/zaz525l ).
-------
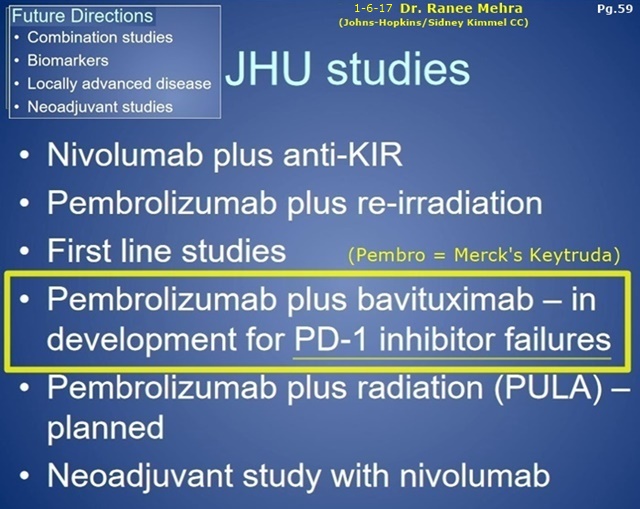
1-6-17 Dr. Ranee Mehra (Johns-Hopkins/SidneyKimmelCC), P.I. for the upcoming NCCN Ph2 Bavi+Keytruda Head&Neck trial. Excerpts from her 1-6-17 talk at GBMC/Greater Balt. MC “H+N Grand Rounds”… I do believe she views this trial is an important part of Johns-Hopkins future anti-cancer direction. Also, look at her Slide #36: “Interferon-y Signature”. Is that an exact tie-in to the newly-revealed AACR’17(4-3-17) Sunrise Biomarker #3 Abstract, “IFN-y Analysis In Blood & Tissue as a Potential Prognostic and/or Predictive Biomarker” (embargoed)? I still totally believe that PPHM has been working with the 3 NCCN Bavi Awardees (Moffitt, MassGEN, JohnsHopkins) to weave in the “chosen” Sunrise Biomarker that is associated with generating “improved outcomes for bavituximab-containing treatments” in future trials.
PDF Link(1-6-17): http://www.gbmc.org/workfiles/HeadNeck/Grand%20Rounds/IO_Therapy_SCCHN02017.pdf
-------
Note: DR. RANEE MEHRA was co-author of ASCO’16, “Biomarkers & Response to Pembro(Keytruda) in Recurrent/Metastatic Head & Neck Cancer” - Conclusion: “The IFN-y signature score was significantly associated with ORR, PFS, and OS (all, P< .001)… PD-L2 & IFN-y signature may be associated with clinical response to Pembro[Keytruda] and may offer addl. strategies to improve prediction of response.” http://meetinglibrary.asco.org/content/165708-176


PPHM’s NCCN#3: Ph2/Progressive Squamous Head+Neck (Bavi+Merck’s Keytruda), JOHNS-HOPKINS(Sidney Kimmel CC) - PI: Ranee Mehra, MD
”Phase II Study of Pembrolizumab[Keytruda] & Bavituximab for Progressive Recurrent/Metastatic Squamous Cell Carcinoma of the Head & Neck" http://tinyurl.com/gutgwb5
Ranee Mehra, MD: Dir., Head & Neck Oncology Therapeutics, Johns Hopkins Medicine https://www.linkedin.com/in/ranee-mehra-34a0467
RANEE MEHRA Disclosures(ASCO’16): GSK, Bayer, BMS, Genentech, Novartis, Mirati Ther.

NCCN Bavituximab Trials Announced 9-6-16 - To Begin "Early 2017" http://tinyurl.com/gutgwb5
...#1: Ph1/HepC-Related Hepatocellular(Liver) (Bavi+RAD+Sorafenib), MOFFITT CANCER CENTER - PI: Jessica Frakes, MD - https://clinicaltrials.gov/ct2/show/NCT02989870
...#2: P1-2/Newly Diag. Glioblastoma (Bavi+RAD+Merck’s Temodar), MASS-GEN. CANCER CENTER - PI: Elizabeth Gerstner, MD
...#3: Ph2/Progressive Squamous Head+Neck (Bavi+Merck’s Keytruda), JOHNS-HOPKINS(Sidney Kimmel CC) - PI: Ranee Mehra, MD
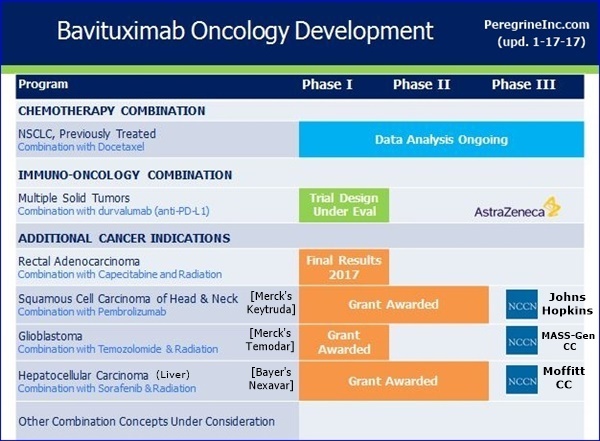
9-9-16/CC/JoeShan: “The 3rd award is for a Phase II study of pembrolizumab [Merck’s Keytruda, anti-PD-1] & bavituximab in Head & Neck Cancer. We are particularly excited about this project, as it will be the 1st clinical trial of bavituximab with a checkpoint inhibitor. In multiple previous preclin. studies, we have observed bavituximab's potential to work synergistically with PD1 inhibitors such as pembrolizumab (Merck’s Keytruda).” http://tinyurl.com/ktrfswj
- - - - - - - - - -
Steve King 9-9-16/CC: “Our collaboration with the NCCN has been an important part of our strategy for advancing the bavituximab clinical program in a cost effective way. We earlier provided NCCN with a $2mm grant to support bavituximab related clinical research with no further financial obligations, and these grant awards represent the outcome of a competitive selection process for the best proposals. These studies will evaluate novel bavituximab combinations in Glioblastoma, Head & Neck Cancer, and Hepatocellular Carcinoma, including an immunotherapy combination [Bavi + Merck’s Keytruda], which is a major focus for advancing the program.” http://tinyurl.com/ktrfswj
- - - - - - - - - -
Steve King 9-9-16/CC/Q&A: “I’m very excited about the combinations that were chosen because the Radiation combination is one that in preclinical studies, as was mentioned during the prepared remarks, has always shown a lot of promise. It’s great to be able to now see that put into a clinical setting in a couple of different clinical trials. And the I-O combinations, as Jeff mentioned during his prepared remarks, is a major focus of ours. So to see a Pembro [Merck’s Keytruda] combination picked as well, we are just really excited that these were the 3 winners out of the NCCN selection process.” http://tinyurl.com/ktrfswj
= = = = = = = = = = = = = =
Apr1-5: AACR 2017, WashDC http://tinyurl.com/zdsbds8 - SUMMARY of PPHM’s 5 AACR’17 ABSTRACTS: [See DETAILS below]
1. MSKCC+PPHM: 4-2-17/1pm #574, “PSTargeting+RAD+AntiPD1 Promotes Anti-Tumor Activity, Melanoma” (same as SITC'16/11-14-16 http://tinyurl.com/js3fca4 )
2. MSKCC+PPHM: 4-3-17/8am #1651, “PSTargeting+Adoptive TCell Transfer (ACT) Eliminates Adv. Tumors w/o Off-Target Toxicities, Melanoma” <=NEW(2nd) Joint MemSloan/PPHM study, see: http://tinyurl.com/h3ylrku
3. PPHM: 4-4-17/8am #3652, “PSTargeting+LAG3+AntiPD1 Significantly Enhances Anti-Tumor Activity, Triple- MBC”
4. IMMUNOVACCINE+PPHM: 4-4-17/8am #3657, ”PSTargeting Enhances Anti-Tumor Activity of a Tumor Vaccine(DepoVax), HPV-Induced Tumor” https://www.imvaccine.com
5. PPHM+VANDERBILT+PRECISIONMEDICINE+PROVIDENCECC: 4-3-17/1pm #CT159/25 (Session: Ph2/3 Clinical Trials in Progress), “IFN-y Analysis In Blood & Tissue as a Potential Prognostic and/or Predictive Biomarker” (abstract embargoed, Sunrise Biomarker #3)
.
.
= = = = = = = = = =DETAILS ON SUNRISE BIOMARKERS #1(B2GPI) & #2(COMPLEMENT/IL-10):
#1 10-10-16/ESMO’16: “B2GPI Biomarker(30%pts) StatSig OS 7.7=>13.2mos.” http://tinyurl.com/hp73njt
10-10-16/PR:
“Peregrine Reports Top-Line and Initial Biomarker Data from Phase III SUNRISE Trial of Bavituximab in Oral Presentation at Eur. Society for Medical Oncology (ESMO) 2016 Congress”
-- Company Has Identified Beta-2 Glycoprotein-1 (B2GP1) as a Biomarker that Correlates with Statistically Significant Improvement in Overall Survival for Patients Receiving the Bavituximab Combination Compared to Chemotherapy Alone
-- Ongoing SUNRISE Trial Biomarker Analysis Expected to Identify Addl. Biomarkers Associated with Patients Benefiting from Bavituximab Treatment that Will Help Guide Program's Future Clinical Development. . .
** "With every clinical trial we conduct, we are constantly reminded of the difficulty involved in treating patients with NSCLC. This continues to prove to be a very challenging cancer to combat and the need for effective treatments remains high," David R. Spigel, MD, CSO and PgmDir. of Lung Cancer Res. at the Sarah Cannon Res. Inst. and one of the lead investigators in the SUNRISE trial. "The findings with regard to B2GP1 that have been collected as part of the ongoing SUNRISE trial data analysis are interesting and support further investigation."
** Peregrine intends to further evaluate the role of B2GP1 levels in response to bavituximab therapy in future clinical trials. The company has filed a new patent application directed to the use of this initial biomarker discovery. Addl. patient sample testing & analysis is ongoing and may result in other biomarkers of importance.
--------------
** Data presented at ESMO’16 demonstrated that patients with pre-treatment B2GP1 levels between 200 and 240 (representing approx. 30% of randomized patients) achieved a statistically significant, 5.5-mo. improvement (13.2 mos. vs. 7.7 mos.) in MOS as compared to patients in the ctl. group with the same range of B2GP1 levels [p = 0.049; HR=.67].
-------------
** "We would once again like to thank all of the patients, clinical investigators and scientists who participated in the SUNRISE trial and have made it possible for us to continue to collect and analyze a range of key data from the study. While we were disappointed with the trial being discontinued earlier in the year, we are excited by the fact that we are beginning to learn important information from the trial through the ongoing biomarker analysis program that will be critical in helping guide the future clinical development of bavituximab," said Joseph Shan, VP/Clin&Reg.Affairs at Peregrine. "It is encouraging that the initial biomarker analysis has identified an important biomarker early in the process and we are optimistic that additional biomarkers associated with improved outcomes for bavituximab-containing treatments will be identified as the analysis continues. We expect to be able to share the emerging data over the coming months at scientific & medical conferences as the more results become available.”
http://investorshub.advfn.com/boards/read_msg.aspx?message_id=125687447
= = = = = = = = = = = = = = = =
#2 12-7-16/WCLC’16(IASLC): “Complement & IL-10 Pathways Id Pts Benefitting from Bavi+Doce” <=CANCELLED/”Anal.Not.Done(IR)”
...Lead author (presenter) is UTSW’s Dr. David Gerber (previously presented Ph2/NSCLC data and Prelim. SUNRISE data at AACR’14). The Senior author is Dr. Rachel E. Sanborn, Co-Dir., Thoracic Oncology Pgm, Robert W. Franz Cancer Res. Center, Earle A. Chiles Res. Inst., Providence CC, Portland, OR. Interestingly, one co-author is Heinrich Roder, CTO of Biodesix, Boulder CO...
ABSTRACT CONCLUSION:
“...Proteomic & correlative approaches identified complement activation and low IL-10 levels as important pathways for predicting improved outcomes of patient treatment with Doce+Bavi, in line with preclinical work on Bavi’s MOA...” [Pts=193, within the 1st subgroup of N=104, a 2nd subgroup isolated: MOS 5.9mos => 12.5mos.]
Dec4-7 2016: “WCLC’16 - IASLC’s 17th World Conf. on Lung Cancer”, Vienna, Austria
http://wclc2016.iaslc.org Pgm: http://wclc2016.iaslc.org/wp-content/uploads/2016/10/WCLC-2016-Poster-Program.pdf
Poster Session with Presenters Present (ID 472) - Track: Advanced NSCLC
12/7/16 2:30-3:45pm David E. Gerber [UTSW], J. Roder, N.L. Kallinteris, L. Horn, G. Losonczy, R. Natale, M. Tang, Heinrich Roder [CTO, Biodesix http://www.biodesix.com/project/heinrichroder ], Joe S. Shan [VP/Clin+Reg], Rachel E. Sanborn [Providence Portland Medical Ctr]
”A Pre-Treatment Serum Test Based on Complement & IL-10 Pathways Identifies Patients Benefiting from the Addition of Bavituximab to Docetaxel”
ABSTRACT Book PDF: http://wclc2016.iaslc.org/wp-content/uploads/2016/12/WCLC2016-Abstract-Book_vF-WEB_revDec12.pdf
ABSTRACT: http://library.iaslc.org/virtual-library-search?product_id=6
BACKGROUND:
SUNRISE, a global, double-bind, Phase III trial of docetaxel (D) plus bavituximab (B) or D plus placebo (P) in previously treated non-squamous non-small cell lung cancer, demonstrated similar overall survival (OS) in both treatment arms. Mass spectrometry and correlative analysis were used to create a test able to identify a subgroup of patients benefitting from the addition of B to D.
METHODS:
Pre-treatment serum samples were available for 197 of the first 200 subjects enrolled in the trial. Mass spectra could be generated for 193 samples using the Deep MALDI method (Duncan et al, ASMS 2013), processed and features (peaks) identified. Mass spectral (MS) features associated with various biological functions were identified using a gene set enrichment analysis approach. Analysis of scores based on these MS feature, subsets indicated that in patients with High Complement Activation outcome depended on IL-10 activation in D+B but not in D+P. A test using the MS features associated with these functions was created to reliably identify a patient subgroup associated with clinical benefit using modern machine learning methods.
RESULTS:
Complement activation, as assessed by a classifier trained using related MS features, was a prognostic factor in both treatment arms, with high activation associated with poorer clinical outcome (OS HR = 0.54, log-rank p = 0.013 for D+B; OS HR = 0.60, log-rank p = 0.040 for D+P). Within the subgroup with high complement activation [N=50 (D+B); N=54 (D+P)], a second classifier using features related to IL-10 activation was able to isolate a subgroup of patients showing numerical benefit from the addition of B [Bavituximab] [median OS 5.9mos.(D+Placebo), 12.5mos.(D+Bavituximab)]. The remaining subgroup showed no benefit from addition of B [MOS 10.4mos.(D+P), 5.6mos.(D+B)]. Blinded validation of the test in the remainder 397 patients randomized in SUNRISE is will be presented.
CONCLUSION:
Proteomic and correlative approaches identified complement activation and low IL-10 levels as important pathways for predicting improved outcomes of patient treatment with D+B, in line with preclinical work on B’s mechanism of action. The test resulting from this work will undergo blinded indep. validation.
- - - - - - - - -
12-20-16/S.Diaz(per Cheynew post #282068): “There was no poster in Vienna. The team was working on a very tight timeframe and, despite their best efforts, couldn’t complete the data analysis in time. Given the crunch, we knew there was a possibility that we wouldn’t meet the timing, so we never issued a PR announcing that we would present. Hence no PR announcing that we did not present.” S.DIAZ/FOLLOWUP: ”Unfortunately, I don’t have a timeline for completion of the analysis or where/when it might be presented. We’ll certainly announce this as soon as we know for sure. Only Joe Shan attended the conference for Peregrine.”
= = = = = = = = = =NOTE:
Ann Oncol (11-8-16/suppl8): ESMO Symposium on Immuno-Oncology, Nov4-6 2016, Lausanne, Switzerland
#30P: “Proteomic Signature Analysis & Application in Clinical Development of the Novel Phosphatidylserine-Targeting Immunotherapy, Bavituximab”
http://annonc.oxfordjournals.org/content/27/suppl_8/mdw525.30
David E. Gerber [UTSW] 1, N.L. Kallinteris 2, L. Horn 3, G. Losonczy 4, R. Natale 5, Heinrich Roder 6 [CTO, Biodesix], M. Tang 7, J. Lai 2, J. Shan 8, Rachel E. Sanborn [9=Providence Portland Medical Ctr]
1 Oncology, UTSW-MC/Dallas
2 Clinical, Peregrine Pharmaceuticals Inc.
3 Oncology, Vanderbilt Ingram CC, Nashville, TN
4 Oncology, Semmelweis Univ., Budapest, Hungary
5 Oncology, Cedars-Sinai M/C, Los Angeles
6 Biodesix, Boulder, CO [CTO, Dr. Heinrich Roder: http://www.biodesix.com/project/heinrichroder ] “Founded in 2005, Biodesix discovers & commercializes cancer tests (diagnostics) that help patients & their doctors make more informed decisions about treatment based on a patient’s unique molecular profile.”
7 Biostatistics, Peregrine Pharmaceuticals Inc.
8 Clinical & Regulatory Affairs, Peregrine Pharmaceuticals, Inc.
9 Thoracic Oncology, Providence Cancer Care, Providence, OR
Aim/Background:
Understanding the multi-dimensional characteristics of cancer is essential to patient selection and treatment planning. Topline results from SUNRISE, a global double-blind Phase III trial of docetaxel + bavituximab (D+B) vs. docetaxel + placebo (D) in previously treated non-squamous NSCLC demonstrated mOS of 10.7mos. in the D+B group and 10.8mos. for the D group, which was unexpectedly different from the assumed 9.1mos. for D+B vs. 7.0mos. used for study powering. VeriStrat, a….[must subscribe]
- - - - - - - - - -
5-31-14 ASCO’14: David Gerber/Joe Shan Poster on Ph3/SUNRISE Trial (#TPS8129) http://tinyurl.com/nv4jloo
Recent CDMO News
- Avid Bioservices Announces Receipt of Deficiency Notice from Nasdaq Regarding Late Form 10-Q • GlobeNewswire Inc. • 03/20/2024 11:00:10 AM
- Form 8-K - Current report • Edgar (US Regulatory) • 03/07/2024 11:30:11 AM
- Avid Bioservices Announces Pricing of Private Placement of Convertible Notes • GlobeNewswire Inc. • 03/07/2024 04:58:48 AM
- Avid Bioservices Announces Proposed Private Placement of Convertible Notes • GlobeNewswire Inc. • 03/06/2024 09:32:07 PM
- Avid Bioservices Announces Certain Preliminary Financial Results for Third Quarter Ended January 31, 2024 • GlobeNewswire Inc. • 03/06/2024 09:31:28 PM
- Form 8-K - Current report • Edgar (US Regulatory) • 03/06/2024 09:30:18 PM
- Form SC 13G/A - Statement of acquisition of beneficial ownership by individuals: [Amend] • Edgar (US Regulatory) • 01/26/2024 09:57:52 PM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 01/13/2024 12:34:35 AM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 01/12/2024 12:39:18 AM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 01/12/2024 12:38:30 AM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 01/12/2024 12:37:38 AM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 01/12/2024 12:36:27 AM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 01/12/2024 12:35:47 AM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 01/11/2024 12:56:02 AM
- Form SC 13G/A - Statement of acquisition of beneficial ownership by individuals: [Amend] • Edgar (US Regulatory) • 01/08/2024 09:32:36 PM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 01/04/2024 12:56:18 AM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 01/04/2024 12:55:07 AM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 01/04/2024 12:53:58 AM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 01/04/2024 12:51:57 AM
- Form SC 13G - Statement of acquisition of beneficial ownership by individuals • Edgar (US Regulatory) • 12/19/2023 09:05:52 PM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 12/19/2023 12:34:08 AM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 12/19/2023 12:33:03 AM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 12/19/2023 12:32:11 AM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 12/19/2023 12:31:12 AM
North Bay Resources Announces Successful Equipment Test at Bishop Gold Mill, Inyo County, California • NBRI • Apr 23, 2024 9:41 AM
Epazz, Inc.: CryObo, Inc. solar Bitcoin operations will issue tokens • EPAZ • Apr 23, 2024 9:20 AM
Avant Technologies Launches Advanced AI Supercomputing Network and Expansive Data Solutions • AVAI • Apr 23, 2024 8:00 AM
BestGrowthStocks.com Issues Comprehensive Analysis of Triller Merger with AGBA Group Holding Limited • AGBA • Apr 22, 2024 1:00 PM
Cannabix Technologies to Present Marijuana Breathalyzer Technology at International Association for Chemical Testing (IACT) Conference in California • BLO • Apr 22, 2024 8:49 AM
Kona Gold Beverages, Inc. Prepares for First Production Run Set to Launch May 17, 2024 • KGKG • Apr 22, 2024 8:30 AM










