Sunday, February 21, 2016 10:57:32 AM
http://oncbiomune.com/
Welcome to OncBioMune Pharmaceuticals Inc.
We are a clinical-stage biopharmaceutical company engaged in the development of novel cancer immunotherapy products, with a proprietary Vaccine Technology that is designed to stimulate the immune system to attack its own cancer while not hurting the patient. Our lead product, ProscaVax™ is scheduled to commence a Phase 2 clinical study in early 2016. OncBioMune also has a portfolio of targeted therapies, some of which are biosimilars to blockbuster drugs. OncBioMune is headquartered in Baton Rouge, LA.
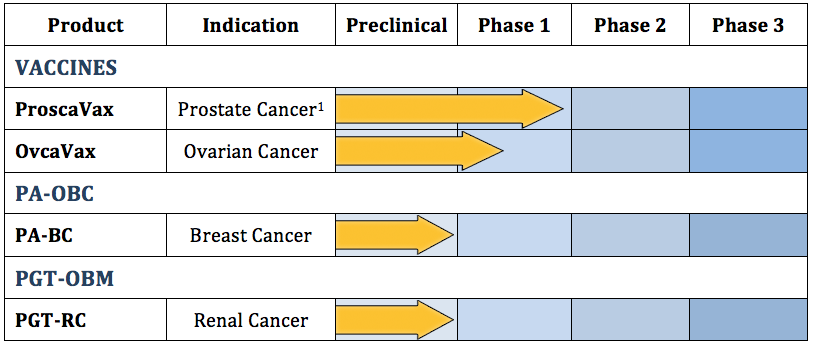
Pipeline:
http://oncbiomune.com/pipeline/
-------------------------------------------
ProscaVax – A Novel Prostate Cancer Vaccine
The Company has developed a therapeutic cancer vaccine for prostate cancer patients using similar techniques developed for breast cancer patients. It is tested and laboratory proven and could become the standard of care for prostate cancer treatment. The Company incorporates scientifically proven and clinically validated treatments for cancer. We utilize patented technology developed and or acquired by OncBioMune. The intellectual property consists of multiple technologies combined with laboratory and clinical procedures that provide new insight into the treatment of cancer. The Company’s proprietary technology provides the necessary tools for the successful treatment of patient’s with a therapeutic vaccine. It is marketable and would be very profitable upon FDA approval.
The Company has data from a phase 1/2 clinical trial of their therapeutic prostate cancer vaccine. Patients with prostate cancer confirmed by biopsy and with an elevated PSA received the vaccine. We enrolled 12 patients in this study. All patients received their initial course of six vaccinations containing prostate specific antigen and biological adjuvant. Serum PSA concentrations were determined before initiating vaccination and 3-4 weeks after the 6th vaccination. Two-thirds of the prostate cancer patients’ PSAs decreased after vaccination. During the trial the prostate cancer patients received no other concurrent therapy (surgery, hormone, radiation, radioactive seeds, chemotherapy), and have additionally received three further vaccinations alternated with low dose IL-2 for the 6 months following the initial vaccinations. The Company developed the protocol for the vaccination of prostate cancer patients using techniques developed for vaccination of breast cancer patients. We are currently in a Phase 1 clinical trial at UCSD Medical School under an IND from the FDA with funding from the US Navy Cancer Vaccine Program. If proven effective clinically, it could become the standard of care for prostate cancer.
Information on the current clinical trial of ProscaVax can be found on the clinictrials.gov at https://clinicaltrials.gov/ct2/show/NCT02058680?term=oncbiomune&rank=1.
-----------------------------
Breast cancers and other epithelial malignancies are antigenic and elicit lymphocyte responses in the autologous host, and patients that express good host immunity to their tumor have better survival. Adequate host immunity is an independent prognostic indicator and those with poor immunity have shorter disease free intervals and shorter overall survival. The Company’s novel approach defines the tumor specific immune status of patients both pre and post immunotherapy that uses autologous tumor antigens to immunize patients. We have the ability to discriminate patients who are immunologically unreactive to tumor antigens and may be generally lymphocyte depressed from those who are tumor antigen reactive and lymphocyte competent. Thus those patients who are unreactive and in need of immunostimulation qualify for vaccination with specific tumor antigen .
We developed our vaccine in 1993 and then immediately began vaccinating patients with depressed immunity. We obtained the first patent on a breast cancer vaccine in the U.S.A. in 1994, and were the first to use the cytokines GM-CSF and IL-2 as biological adjuvants. Appropriate patients are vaccinated in the adjuvant setting and patients with advanced disease are treated with a combined chemo-immunotherapy protocol. Some of these patients have had dramatic responses.
The Company is presently devoting much research to the tumor stroma and microenvironment for a better understanding of tumor escape mechanisms. This will allow us the ability to better attack the tumor with specific adaptive immunotherapy. This work is in progress and early results are producing exciting results.
=======================================
OncBioMune Pharmaceuticals CEO Dr. Jonathan Head presented at the 2016 BIO CEO & Investor Conference on February 9, 2016 at the Waldorf Astoria in New York City. Dr. Head’s presentation provided a corporate overview of OncBioMune and provided details on the company’s promising pipeline of drugs in development, including ProscaVax, OncBioMune’s novel vaccine in clinical trials as a new therapy for early stage prostate cancer patients.
http://oncbiomune.com/oncbiomune-bio-ceo-and-investors-conference-2016/
=========================================
SEC Filings
http://www.sec.gov/cgi-bin/browse-edgar?action=getcompany&CIK=0001362703&type=&dateb=&owner=include&count=40
=============================================
Quotes
http://finance.yahoo.com/q?s=obmp&ql=1
http://finra-markets.morningstar.com/MarketData/EquityOptions/detail.jsp?query=22:0P0000K6PP
============================================
"Perfection is not attainable, but if we chase perfection we can catch
excellence." Vince Lombardi
Do your research! Play the TA. All posts are my opinion.
Recent THER News
- Form 8-K - Current report • Edgar (US Regulatory) • 03/06/2024 09:29:33 PM
- Form 10-Q - Quarterly report [Sections 13 or 15(d)] • Edgar (US Regulatory) • 02/20/2024 08:35:09 PM
- Form 10-K/A - Annual report [Section 13 and 15(d), not S-K Item 405]: [Amend] • Edgar (US Regulatory) • 02/15/2024 10:28:54 PM
- Form NT 10-Q - Notification of inability to timely file Form 10-Q or 10-QSB • Edgar (US Regulatory) • 02/15/2024 08:00:37 PM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 02/03/2024 01:10:18 AM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 01/26/2024 11:28:46 PM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 01/26/2024 11:17:03 PM
- Form NT 10-K - Notification of inability to timely file Form 10-K 405, 10-K, 10-KSB 405, 10-KSB, 10-KT, or 10-KT405 • Edgar (US Regulatory) • 12/29/2023 04:26:15 PM
- Form 3 - Initial statement of beneficial ownership of securities • Edgar (US Regulatory) • 12/19/2023 09:51:04 PM
- Form 10-Q - Quarterly report [Sections 13 or 15(d)] • Edgar (US Regulatory) • 08/21/2023 08:08:50 PM
- Form NT 10-Q - Notification of inability to timely file Form 10-Q or 10-QSB • Edgar (US Regulatory) • 08/15/2023 08:46:06 PM
- Form 8-K - Current report • Edgar (US Regulatory) • 06/29/2023 08:01:16 PM
North Bay Resources Announces Successful Equipment Test at Bishop Gold Mill, Inyo County, California • NBRI • Apr 23, 2024 9:41 AM
Epazz, Inc.: CryObo, Inc. solar Bitcoin operations will issue tokens • EPAZ • Apr 23, 2024 9:20 AM
Avant Technologies Launches Advanced AI Supercomputing Network and Expansive Data Solutions • AVAI • Apr 23, 2024 8:00 AM
BestGrowthStocks.com Issues Comprehensive Analysis of Triller Merger with AGBA Group Holding Limited • AGBA • Apr 22, 2024 1:00 PM
Cannabix Technologies to Present Marijuana Breathalyzer Technology at International Association for Chemical Testing (IACT) Conference in California • BLO • Apr 22, 2024 8:49 AM
Kona Gold Beverages, Inc. Prepares for First Production Run Set to Launch May 17, 2024 • KGKG • Apr 22, 2024 8:30 AM









