| Followers | 371 |
| Posts | 16703 |
| Boards Moderated | 3 |
| Alias Born | 03/07/2014 |
Saturday, January 09, 2016 4:56:37 PM
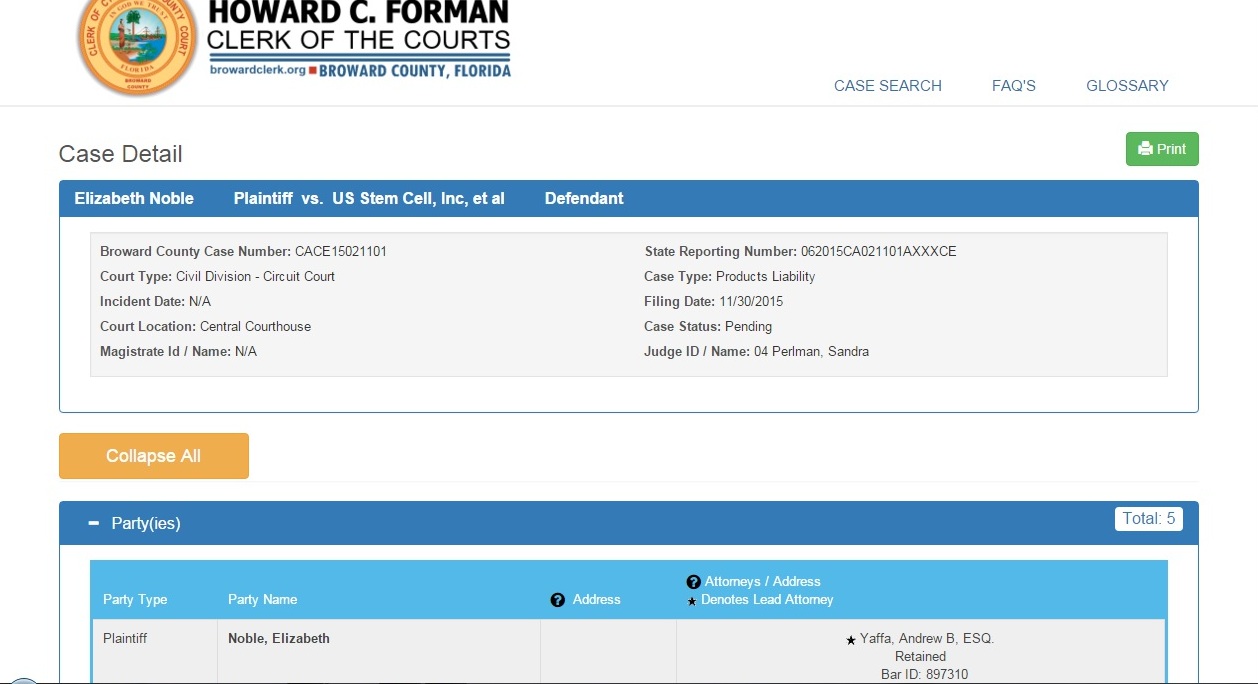
Filed 11/30/2015, Broward County Court in Florida
HOLY FREAKING COW. I was wondering when their little "clinic" thingy and their NON FDA approved so called "treatments" was gonna get um in some hot water- well, looks like it's happened for real.
U.S. Stem Cell and U.S. Stem Cell Clinic are being sued by a plaintiff, big time, for all sorts of alleged damages relating to claims apparently about being "treated" at the ole little stem cell clinic thingy and with non FDA approved promised "products" made by U.S. Stem Cell and then promised to be "treatments" that did not work, etc.
Here it is on the Broward County Clerk of the Court government run site:
http://www.clerk-17th-flcourts.org/Web2/CaseSearch/Details/?caseid=ODM0MzA5Nw%3d%3d-BwUDbrS1LbA%3d&caseNum=CACE15021101&category=CV
NOT LOOKING GOOD IMO. U.S. Stem Cell is already cash broke for all intents and purposes- what's this mess gonna due to um now? Where they gonna get the cash to pay more freaking lawyers after they just recently settled the Brenda Leonhardt case brought against um last year- that they settled and now owe her payments on?
Wowza? Not surprise though- I figured something like this was coming, seeing how they wanted to start dabbling in NON FDA approved so called "treatments/cures/products" etc.
Here's the entire complaint filing- it's lengthy and full of all kinds of "nuggets" about this new case, filed on 11/30/2015:
http://www.clerk-17th-flcourts.org/Web2/WebForms/Document.aspx?CaseID=ODM0MzA5Nw%3d%3d-BwUDbrS1LbA%3d&CaseNumber=CACE15021101&Style=Elizabeth+Noble%0a++++++++Plaintiff%0a+vs.+%0a%0aUS+Stem+Cell%2c+Inc%2c+et+al%0a++++++++Defendant&FragmentID=MTY1NjY4MjY%3d-Xu2inXiVuH0%3d&DtFile=11/30/2015&DocName=Request+for+Production&PgCnt=4
http://www.clerk-17th-flcourts.org/Web2/WebForms/Document.aspx?CaseID=ODM0MzA5Nw%3d%3d-BwUDbrS1LbA%3d&CaseNumber=CACE15021101&Style=Elizabeth+Noble%0a++++++++Plaintiff%0a+vs.+%0a%0aUS+Stem+Cell%2c+Inc%2c+et+al%0a++++++++Defendant&FragmentID=MTY1NjY3ODU%3d-Xk5ZbqvBbkQ%3d&DtFile=11/30/2015&DocName=Complaint+(eFiled)&PgCnt=38
How the FREAK does this company not release a SEC filing for a major "material event" as being sued for your new "clinic" thingy? Seems odd to me they don't utter a word about this to their shareholders?
The crux of the case from a first read- and it's a whopper, is that the plaintiff claims to be damaged from FALSE PROMISED TREATMENT OF EYE MACULAR DEGENERATION using an un-approved, non proven "stem cell treatment" as peddled by U.S. Stem Cell and their little clinic thingy. Biheart has/had NO EXPERIENCE injecting cells into people's eyes that I've ever heard of or read in their SEC filings? Good gawd, they're promoting their cells for EYE INJECTIONS NOW?
That's an incredibly specialized area of clinical trials going on at present via OCATA Therapeutics for example, taking um YEARS of research just to get through a phase I and with a world recognized "genius" M.D. named Dr Robert Lanza heading up their research (with another world famous doctor at UCLA) looking as therapies/solutions for eye/macular degeneration treatments using stem cells. What the FREAK is Bioheart doing now involving the human eye of all things? Good gawd almighty that just makes NO SENSE TO ME, NONE????
HOLY COW the charges are serious- such as a medical product made by U.S. Stem Cell "reaching the patient/plaintiff in a condition DANGEROUS TO HER DUE TO MANUFACTURING DEFECTS" in one of the many, many, many counts in this suit. WHAT A STUNNER- this is gonna be wild to see where this goes?
Complaint also alleges that U.S. Stem Cell made and sold these medical "products/treatments" that have NO PROVEN OR KNOWN THERAPEUTIC VALUE"
WOWZA again. It's a real lengthy complaint- like 38 freaking pages. HOLY COW.
Complaint talks about U.S. Stem Cell "down playing" known "risks to the patients" and not "labeling a medical product correctly" and thus causing the patient damage !! The words BLINDNESS, PAIN, PERMANENT DAMAGES TO THE EYE are specifically mentioned in the allegations/charges of the complaint.
There's a damages summary page that lists the following (Page 37 of 38 of the complaint filed w/ the court):
a) BODILY INJURY
b) PAIN AND SUFFERING
c) DISABILITY
d) DISFIGUREMENT
e) LOSS OF THE CAPACITY FOR THE ENJOYMENT OF LIFE
f) AGGRAVATION OF PRE-EXISTING CONDITIONS
g) MEDICAL AND HOSPITAL CARE EXPENSES
h) LOSS OF EARNINGS
i) REHABILITATION EXPENSES
j) MENTAL DISTRESS
Demanding TRIAL BY JURY
WOWZA HOLY COW, this is serious stuff IMO. I mean big league medical/product liability and malpractice type stuff. It involves the plaintiff's FREAKING EYE and VISION, can you imagine that? What's this gonna cost ole CASH BROKE Bioheart/U.S. Stem cell to defend as they're waiting on Magna dilution shares to get registered for sale in another round of mass dilution per the SEC filed S-1, recently, LOL???
Gawd, what a train wreck of a "business/company"???
Here is the plaintiff's law firm, the attorney signing the complaint is Andrew Yaffa:
http://www.grossmanroth.com/our-team/andrew-b-yaffa/
He's won over $250 MILLION in awards for his clients in his career. HOLY FFREAKING COW ! U.S. Stem Cell's entire market cap is what, like about $1 MILLION lousy bucks right now and they finished the last qtr with like less than a $100K cash in the bank, LOL !!
http://www.grossmanroth.com/
Andrew Yaffa won an award recently as a TOP MEDICAL MALPRACTICE ATTORNEY- HOLY FREAKING COW. This dude looks like a heavy hitter, as does this law firm. This ain't no yellow pages ambulance chasing firm of amateurs IMO.
http://www.grossmanroth.com/news/andrew-yaffa-gary-cohen-selected-best-lawyers-2016-medical-malpractice-law-plaintiffs-lawyer-year/
Good gawwd- look at the size of this plaintiff LAW FIRM- they have mega staff, their own medical investigators, NUMEROUS attorneys, etc. U.S. Stem Cell has what, like FOUR total "employees" LOL !! Look at this law firm they're up against- I count ELEVEN attorneys on their staff and then TEN other firm personnel listed. TWENTY ONE staff at this law firm suing lil ole U.S. Stem Cell?
The line that always stood out to me in Bioheart's own SEC filings (U.S. Stem Cell whatever) - was about how they supposedly HAD NO APPROVED PRODUCTS to sell/market and thus did NOT carry product liability insurance, despite claiming in other parts of the same SEC filing to be SELLING FREAKING "kits" and "treatments" etc LOL?? NEVER COULD MAKE SENSE OF THAT.
U.S. Stem Cell/Bioheart last filed 10-K:
http://www.sec.gov/Archives/edgar/data/1388319/000114544315000378/bioheart_10k.htm
PAGE 32:
"
We do not currently have product liability insurance because none of our product candidates has yet been approved for commercialization. While we plan to seek product liability insurance coverage if any of our product candidates are sold commercially, we cannot assure you that we will be able to obtain product liability insurance on commercially acceptable terms, if at all, or that we will be able to maintain such insurance at a reasonable cost or in sufficient amounts to protect against potential losses.
Claims may be made by consumers, healthcare providers, third party strategic collaborators or others selling our products if one of our products or product candidates causes, or appears to have caused, an injury. We may be subject to claims against us even if an alleged injury is due to the actions of others. For example, we rely on the expertise of physicians, nurses and other associated medical personnel to perform the medical procedures and processes related to our product candidates. If these medical personnel are not properly trained or are negligent in using our product candidates, the therapeutic effect of our product candidates may be diminished or the patient may suffer injury, which may subject us to liability. In addition, an injury resulting from the activities of our suppliers may serve as a basis for a claim against us.
32
We do not intend to promote, or to in any way support or encourage the promotion of, our product candidates for off-label or otherwise unapproved uses. However, if our product candidates are approved by the FDA or similar foreign regulatory authorities, we cannot prevent a physician from using them for any off-label applications. If injury to a patient results from such an inappropriate use, we may become involved in a product liability suit, which will likely be expensive to defend.
These liabilities could prevent or interfere with our clinical efforts, product development efforts and any subsequent product commercialization efforts, all of which could have a material adverse effect on our business."
This has GOT TO BE A MAJOR, MAJOR hit to this little cash poor company? AND, they're sitting here waiting on Magna dilution shares to get SEC approved on that S-1 filiong, 340K of um, just for a lousy $200K or so more of survival cash. Defending a case like this can EASILY cost $200K or more IMO. Will the convertible debt, toxic loan dudes even loan to a company involved now in a major product liability suit? Even the toxic lenders have limits it seems- if they smell the company might get BK'd from losing a legal case like this, are they going to be willing to loan um high risk dilution-for-cash money still?
This is a stunner IMO. And the company hasn't said a freaking PEEP about it to the shareholders? SHAZAM, why am I not surprised? No "blog" or fantastic sounding little "PR" about this little nugget of "news"- a freaking major product liability suit for their NON FDA APPROVED "kits" and "cells" and their little new stem cell "clinic" thingy being right smack in the middle of it all?
Wow IMO.
Posts contain only my amateur opinions, personal views and thoughts. I discuss stocks as a hobby only. Always do one's own due diligence before investing.
VPR Brands LP Reports Record Annual Financial Performance for Fiscal Year 2023 • VPRB • Apr 19, 2024 11:24 AM
Coinllectibles' Subsidiary, Grand Town Development Limited, Acquires Rare Song Dynasty Ceramics Worth Over USD28million • COSG • Apr 18, 2024 8:03 AM
ILUS Provides Form 10-K Filing Update • ILUS • Apr 17, 2024 9:54 AM
Glucotrack Announces Expansion of Its Continuous Glucose Monitoring Technology to Epidural Glucose Monitoring • GCTK • Apr 17, 2024 8:00 AM
Maybacks Global Entertainment To Fire Up 24 New Stations in Louisiana • AHRO • Apr 16, 2024 1:30 PM
Cannabix Technologies Begins Certification of Contactless Alcohol Breathalyzer, Re-Brands product series to Breath Logix • BLOZF • Apr 16, 2024 8:52 AM









