Register for free to join our community of investors and share your ideas. You will also get access to streaming quotes, interactive charts, trades, portfolio, live options flow and more tools.
New here, Whats the deal with DARA?
yup, that mitch cat sure knows whats up, eh? like, stock killed, dead! MTP. hahahahahaha
GoodPlenty, Yeah the surfdude is here, and been scarfing some additional shares since I was turned on to the Mitch's personal email. I found the cat in blog land tied to some other hefty investments and reached out. That cat had the scoop all along and knew to load, load long and he's not selling until the major news, so he says.
Me well I hold a fair amount of shares and willing to wait and see how the ADR thing shakes out.
Any thoughts in DARA land??
surfdude, still hanging in there are you? I still haven't voted my share as of yet. Looking at that $1.08 floor not exactly floating my boat. I have been away from the board since I left the mod. possition, just so disgusted with managements mismanagement.
Only hope is how the ADRs will trade after the transaction closes.
Would love to hear from Mitch as well on that topic.
DARA Biosciences Arbitrage Opportunity
http://portal.geoinvesting.com/geoarticles/1221/dara_biosciences_arbitrage_opportunity
Well I guess that the Mitch was right.....the DEF14 form was filed about the vote for the merger. Man all those people over at YMB kept bashing that cat for years saying he didn't know a thing, yet he's been consistantly correct everytime I have checked. Wow.....now let's get the merger completed
Rumor has it that the cat (Mitch) over at YMB was tweeting or blogging to someone that last week in Oct news of merger will be released. Wait and see I guess
Well at least you're not calling me short. Lol. :)
I'm glad you've walked away with a different perspective. I respect that. I put the information out there so that I don't ever feel guilty about not sharing what I uncovered incase it falls flat. Also don't want folks to expect me to keep coming back to respond. :)
Go Dara. :)
i'm not saying you are wrong.. i'm not saying i don't understand..i am saying you have a different perspective.
Good Luck.
not sure of any buzz ....however the chart is shaping up
Long time DARA shareholder here. Why the spike in price? Pleased as punch but not sure what the buzz is here..
Will look at it this weekend. I'm still holding this one too. Buying on dips as it's a semi-confirmed move to $1.20. Nice place to park money. :)
nope your buy was good given the near term price appreciation i expect IMO. why would i buy if i didn't think so but what do i know? ;)
i'm just saying do some deep DD this weekend.
sometimes you have to take profits on an issue that you have profits on and invest in an another issue that has more near to medium term potential.
i am serious on my belief and potential of the other issue or i wouldn't be so heavily invested. if it breaks the 1.35 mark then do consider a heavier investment.
edit: actually if it bounces off the 1.35 mark again buy all that you can.
That's terrible news hopes and wishes for recovery are with you Rambo..
Hope you get better rambo
For all those who followed ,Rambo trader. I got word that he was paralyzed in a horrible bike accident. Wishing you the best bud
Is this thing DEAD????
who turned out da lights???
'click'
i think DEAD line... is the operative word, lol
when is the deadline to vote on the merger?
waiting three years is called investing for a return. Day trading is more like gambling and usually lose more than gain fact.
Like I said in the end we will all be dead. Waiting 3 years for such a minimal gain makes no sense, the stock market is vast and you could easily make more money elsewhere. Time is money. Let your money work for you and not you for the money.
Me also 24 - 48 "money in the bank"....
Long term for me is months +
2018 sounds about right according to the payout for CVG's and probable growth of MTPH.L so let's just call it money in the bank. When the time comes to need it, it'll be there waiting "with interest".....surfs up party on.
It depends how long, "long term" is because as the saying goes in the long term we will all be dead.
I bought back in. :)
The company value is higher than the value reflected per the closed deal IMO. I believe this will pay off for long term investors
She'll surf higher and sooner....The market knows more, then whats on the table. Also that cat Mitch has pegged this on YMB and got it right.
All the rocks fall in the right place this is 1.20 in about 2 months.
re: 425 transcript.
More % than my savings account makes. Understand this is the market and anything can happen. I'm liking the odds obviously.
Bagholder? My average is about 93 cents
To the contrary, DARA insiders only holds about 15% of OS. Institution holders would likely side with retails to fight for a higher buyout price.
ash111, Great call on this one! Thanks for tip!
Eagle1
I see these often. In this case the best outcome would be to pressure DARA into seeking a higher merger price based on the potential. Sometimes a higher price is offered but not often, and without circumstance. No case imo. And don't thing retail has enough votes to force a higher price.
Thoughts on the "investigation"?
Supposed to get a fraction of MTPH shares per share of DARA coming out to about $1.20 relative value per share of DARA...something like that.
Merger I believe.
So what's the deal here?? I'm looking for entry at .80
ATM at work here guys
Good luck
hits above ask
did you check this Presentation?.the buyer got a huge deal here imo-link back.
You know me and my typos. I never got into the typing with two fingers on my phone. Lol. ;)
Thanks i will check it out but that wasn't the one that was misspelled :). umm ... GO DARA!
if you got in the misspelled ticker in the low .20s you did good otherwise i'd be very careful. i had that one more than a year ago and know it pretty well don't know the other one but i'll check it out. i had this one (DARA) about a year ago and got out with small loss but i have kept an eye on it.
Agree. Give it the weekend to build some momo for Monday. ![]()
|
Followers
|
118
|
Posters
|
|
|
Posts (Today)
|
0
|
Posts (Total)
|
6805
|
|
Created
|
10/30/06
|
Type
|
Free
|
| Moderators | |||
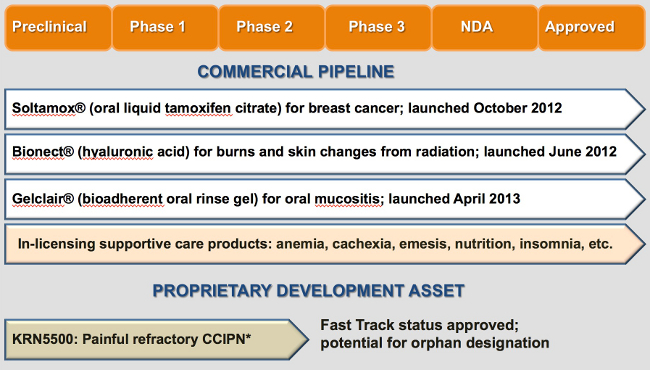

Soltamox® (tamoxifen citrate) is the only oral liquid formulation of tamoxifen available in the US and is an important option for breast cancer patients who have difficulty adhering to tablet therapy.
Tamoxifen is widely used to treat estrogen-receptor positive breast cancer and patients at high risk for the disease. In the US, over 230,000 new cases of breast cancer are diagnosed each year, and over 1.8 million prescriptions are written for tamoxifen.
While tamoxifen is an effective therapy, it must be taken for up to five years in order for the full benefits to be realized. A number of well designed studies indicate long term adherence to treatment is less than optimal, with up to 50% of patients discontinuing treatment earlier than they should. To address the need for improved adherence, Soltamox was developed to offer breast cancer patients a new option for daily compliance. Patients who may benefit from Soltamox include those who prefer a liquid versus a pill, patients who have swallowing difficulties and patients who may benefit from a change in formulation. For patients with intolerance to gluten or lactose, Soltamox is both gluten and lactose free.
Soltamox is available as a sugar-free oral solution (equivalent to 10mg/5mL). Each bottle contains 150 mL.
DARA has an exclusive license to market Soltamox® in the U.S. from Rosemont Pharmaceuticals, LTD, a UK-based manufacturer and marketer of oral liquid medicines. Soltamox is patent-protected through 2018
For additional information on Soltamox visit: http://soltamox.com/
For important safety information and warnings please click here.

Gelclair® is a bioadherent oral rinse gel indicated for the management and relief of pain associated with oral mucositis, a condition characterized by erythema (redness, swelling) and painful oral lesions. Oral mucositis frequently occurs in cancer patients, a result of chemotherapy or radiation therapy. It is estimated over 400,000 cases of therapy-induced oral mucositis occur annually, and is seen most often in patients receiving radiation therapy for head and neck cancer, and patients receiving high-dose chemotherapy conditioning prior to hematopoietic stem cell transplantation (HSCT). Left untreated, oral mucositis can lead to intense pain, impact patients’ ability to swallow food and liquids, cause weight loss, delay cancer treatment and increase treatment costs.
Gelclair is a unique oral gel whose key ingredients are polyvinlypyrrolidone (PVP) and sodium hyaluronate (Hyaluronic Acid). Gelclair is easily applied, via an oral rinse, and provides rapid and effective relief from pain, by creating a protective coating over the oral mucosa. This coating, or barrier, alleviates irritation of exposed nerves in the ulcer areas, as well as moisturizing the tissue. Patients experience relief from pain and are also able to eat and drink more easily.


Gelclair is the most clinically studied oral gel in the US, with studies supporting:
For additional information on Gelclair visit: http://gelclair.com/

DARA obtained a license from Innocutis Holdings for the exclusive rights to market Bionect® (hyaluronic acid sodium salt, 0.2%) in both radiation and oncology. Bionect treats burns and other skin changes (radiation dermatitis) associated with radiation treatment. Bionect is a patent-protected low-molecular-weight hyaluronic acid (LMW-HA) cream or gel which penetrates to the dermal layer. In a randomized double-blind placebo-controlled study of 134 radiation patients, the low molecular weight hyaluronic acid (LMW-HA) formulation Bionect cream was shown to significantly reduce the incidence of high-grade skin reactions, delay their onset & reduce their severity. (Ligouri V. et al, Radioth Oncol. 1997; 2:155-161)
For additional information on Bionect visit: http://bionect.com/
KRN5500 is a non-narcotic, non-opioid, intravenously administered new chemical entity (NCE) in development for painful chronic chemotherapy induced peripheral neuropathy (CCIPN). DARA is currently seeking a development and commercialization partner for KRN5500.
KRN5500 has successfully completed a Phase 2a proof of concept study in patients with end-stage cancer, meeting its primary endpoint of pain reduction relative to placebo (p = 0.03). Safety results from the study included clinically important gastrointestinal side effects, but no other major safety concerns were found at doses that were much lower than those studied in earlier Phase 1 oncology trials completed under a National Cancer Institute (NCI) investigational new drug (IND) application for treatment of solid tumors.
In 2011, the U.S. Food and Drug Administration (FDA) designated KRN5500 a Fast Track Drug Development Program. The Fast Track initiative is designed to facilitate development and expedite review of new drugs that demonstrate potential for addressing unmet medical need in serious or life threatening conditions. The purpose of the FDA program is to provide important drugs to the patient earlier. Under a separate FDA initiative, DARA has submitted a request for orphan drug designation for treatment of patients with painful CCIPN. Orphan designation provides extended market exclusivity, tax benefits, and waiver of PDUFA fees.
In addition to DARA’s primary development program for painful CCIPN, a sub-category of neuropathic pain that is particularly devastating to patients that have been treated with powerful and life-saving chemotherapeutic agents, a Clinical Trial Agreement with NCI provides an opportunity to collaborate on secondary exploratory studies that would focus on additional aspects of painful and chronic CIPN.
DARA is a specialty pharmaceutical company focused on the development and commercialization of oncology treatment and supportive care products. DARA holds the exclusive U.S. marketing rights to Soltamox®, a novel oral liquid formulation of tamoxifen citrate, which is widely used in the treatment and prevention of breast cancer. Soltamox is the only FDA-approved oral liquid version of tamoxifen. DARA has exclusive U.S. rights to Soltamox through a license from Rosemont Pharmaceuticals, Ltd.
In September 2012, DARA entered into an exclusive agreement with the Helsinn Group of Switzerland for U.S. commercial rights to Gelclair®, an FDA-cleared product for the treatment of oral mucositis.
Additionally, in June 2012, DARA launched its first product, Bionect®, a topical treatment for skin irritation and burns associated with radiation therapy. DARA has rights to market Bionect in the US oncology/radiology markets under license from Innocutis.
DARA is currently developing a cancer-support therapeutic compound, KRN5500, for the treatment of painful chronic chemotherapy induced peripheral neuropathy (CCIPN) in patients with cancer. This product is an excellent fit with DARA’s strategic oncology focus. KRN5500 has successfully completed a Phase 2a clinical trial, and has been designated a Fast Track Drug by the United States Food and Drug Administration. DARA is working with the National Cancer Institute (NCI) to design an additional clinical trial under joint DARA-NCI auspices while continuing further Phase 2 development. DARA is currently seeking a development and commercialization partner for KRN5500.
We have an intellectual property estate generated by license agreements as well as internal development. The patents granted related to each of our commercial products and development-stage programs is shown in the following table.
Product / Program | Intellectual Property |
| Soltamox® | US Patent 6,127,425 licensed from Rosemont Pharmaceuticals, Inc. |
| Gelclair® | US Patent 6,828,308 licensed from Helsinn. |
| Bionect® | US Patent 5,925,626 licensed from Innocutis. |
| KRN5500 Program | US Patents 5,905,069, 7,196,071, 7,375,094, 7,632,82 and related foreign patents licensed from Massachusetts General Hospital. Additional provisional and non-provisional patent applications pending (internal development). |
| DB959 Program | US Patents 6,828,335, 7,476,742, 7,714,004, 7,906,653, 7,592,361, 7,964,622 and related foreign patents licensed from Bayer Pharmaceuticals. Additional non-provisional patent applications pending (licensed subject matter and internal development). |
DARA has comprehensive commercial coverage across the national oncology market through a series of agreements with a number of specialty pharmacy providers, leading group purchasing organizations (GPOs), retail partners, reimbursement experts, and an industry-leading third-party logistics provider. As part of an integrated national network with annual sales of over $1 billion in cancer therapeutics, DARA has significant commercial scale and capabilities. Its distribution network consists of more than 45,000 retail pharmacies, mail order pharmacies, and long-term care facilities. This provides DARA with established reimbursement and logistics expertise, as well as partnering opportunities with more than 300 sales and marketing personnel uniquely focused on oncology and oncology support products. This comprehensive network of partners is rare if not unique among companies in the oncology supportive care area and provides DARA a strong foundation for product introductions into the oncology market.
Our business development efforts are focused on actively identifying in-licensing opportunities to expand our oncology supportive care focus product portfolio. Areas of interest include anemia, cachexia, emesis, nutrition and pain.
We are actively seeking interested partners for the development and commercialization of KRN5500. KRN5500 is a non-narcotic, non-opioid, intravenously administered new chemical entity (NCE) in development for painful chronic chemotherapy induced peripheral neuropathy (CCIPN). KRN5500 has successfully completed a Phase 2a proof of concept study in patients with end-stage cancer, meeting its primary endpoint of pain reduction relative to placebo (p = 0.03).
DB959 is a PPAR-delta/gamma agonist which has been in clinical development for Type 2 diabetes. It was tested in Phase 1 clinical studies that demonstrated a good safety profile and provided strong evidence of pharmacologic activity in healthy volunteers. This program is currently available for out licensing.
If you are interested in exploring business development opportunities with DARA, please contact:
David Benharris
Vice President of Sales, Marketing & Business Development
919-861-0158
dbenharris@darabio.com
DARA has implemented a targeted and focused commercial approach to effectively reach oncologists in the United States. The primary strategy is to deploy a team of experienced Regional Business Directors (“RBD’s”) that have significant experience in all commercial aspects of the niche pharmaceutical industry, targeting the high prescribing oncologists. The RBD’s team affords DARA coverage across the US. In addition, strategic partnerships with specialty pharmacy groups will allow significantly increased reach and frequency to key customers, both physicians and patients.
| Volume | |
| Day Range: | |
| Bid Price | |
| Ask Price | |
| Last Trade Time: |
