Register for free to join our community of investors and share your ideas. You will also get access to streaming quotes, interactive charts, trades, portfolio, live options flow and more tools.
Thanks for the update Renee.
For those shareholders that held the stock, it is now a waiting game. Hopefully, Griffons will obtain FDA approval and is able to sell the Aradigm products it purchased as Aradigm receives royalties from those sales. It will likely be years before we hear anything new on this stock, so best to file this stock away and forget about it. The projection was that shareholders would receive $0-18m.
ARDMQ BK PLAN effective. All shares cancelled.
https://otce.finra.org/otce/dailyList?viewType=Deletions
Aradigm Receives Feedback Following an Oral Explanation With the European Medicines Agency
Press Release | 10/21/2019
Aradigm Corporation (OTCQB: ARDM) (“Aradigm” or the “Company”) today announced that following a recent Oral Explanation, it has received feedback that a negative opinion is likely to be received in November 2019 from the CHMP (Committee for Medicinal Products for Human Use), a committee of the European Medicines Agency (EMA), for the centralized marketing authorization application (MAA) for Linhaliq as a treatment for non-cystic fibrosis bronchiectasis (NCFBE) patients with chronic lung infections with Pseudomonas aeruginosa (P. aeruginoa).
On February 15, 2019, Aradigm filed a petition for bankruptcy protection under Chapter 11 of the United States Bankruptcy Code in the United States Bankruptcy Court for the Northern District of California, Case no. 19-40363. Information regarding Aradigm’s bankruptcy case and Aradigm’s Monthly Operating Reports may be obtained from the Bankruptcy Court.
The Company has engaged Evergreen M&A Partners, LLC to facilitate the sale of substantially all of Aradigm’s assets, including its core intellectual property rights, and expects to secure initial bids for those assets within the next 30 to 60 days. Any final sale transaction will be subject to Bankruptcy Court review and approval following an auction process. The proceeds from the sale would be distributed to satisfy the claims of the Company’s creditors, also subject to Court approval. Remaining assets, if any, would then be distributed to the Company’s stockholders.
About Aradigm
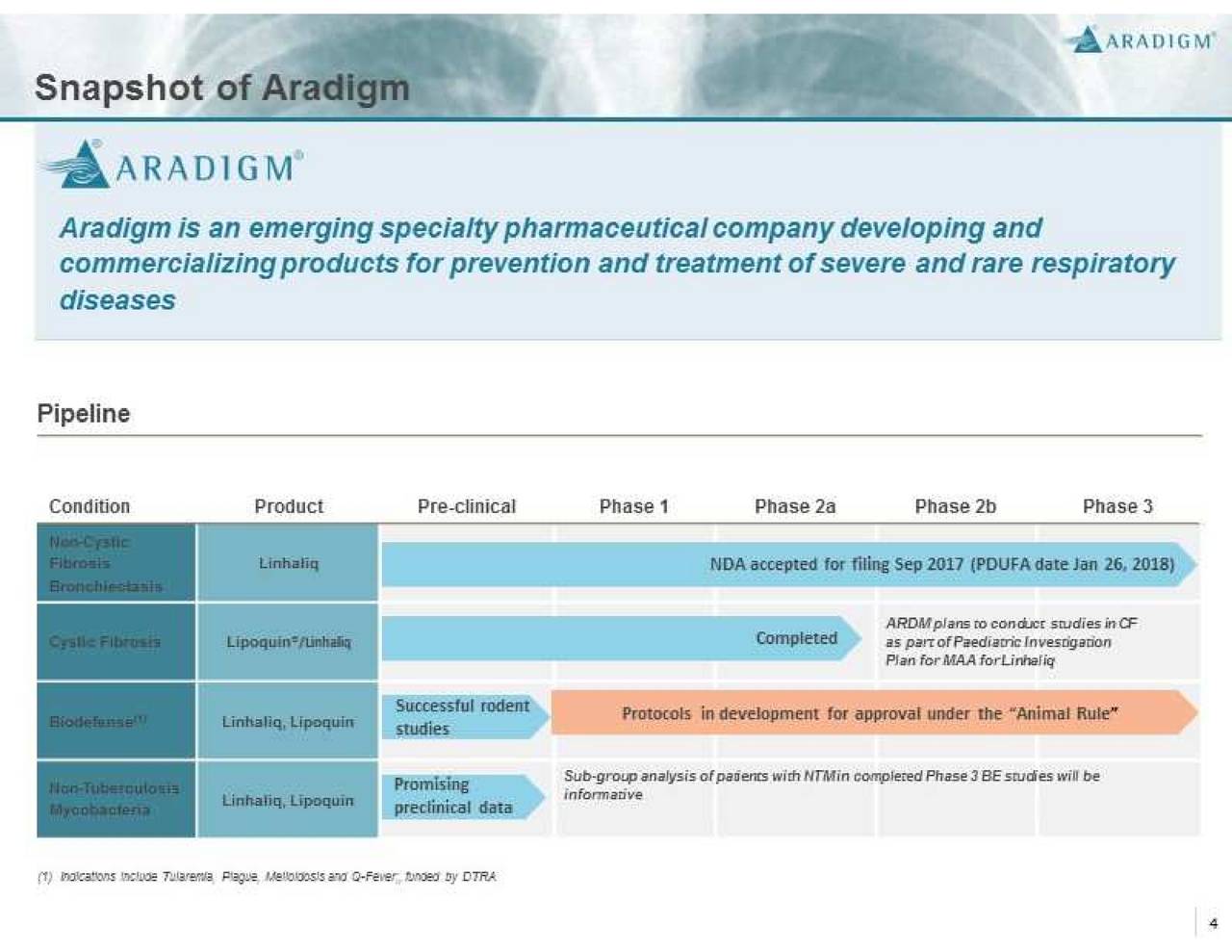
Aradigm is an emerging specialty pharmaceutical company focused on the development and commercialization of drugs for the prevention and treatment of severe respiratory diseases. Aradigm remains confident in the efficacy, safety and quality of Apulmiq (US) / Linhaliq (EMA). Aradigm is currently in Phase 3 development of Apulmiq/Linhaliq (an investigational proprietary formulation of ciprofloxacin for inhalation) for the treatment of patients with NCFBE and chronic lung infection with P. aeruginosa. Aradigm's inhaled ciprofloxacin formulations are also product candidates for treatment of patients with cystic fibrosis and non-tuberculous mycobacteria, and for the prevention and treatment of high threat and bioterrorism infections, such as inhaled tularemia, pneumonic plague, melioidosis, Q fever and inhaled anthrax.
About Non-Cystic Fibrosis Bronchiectasis
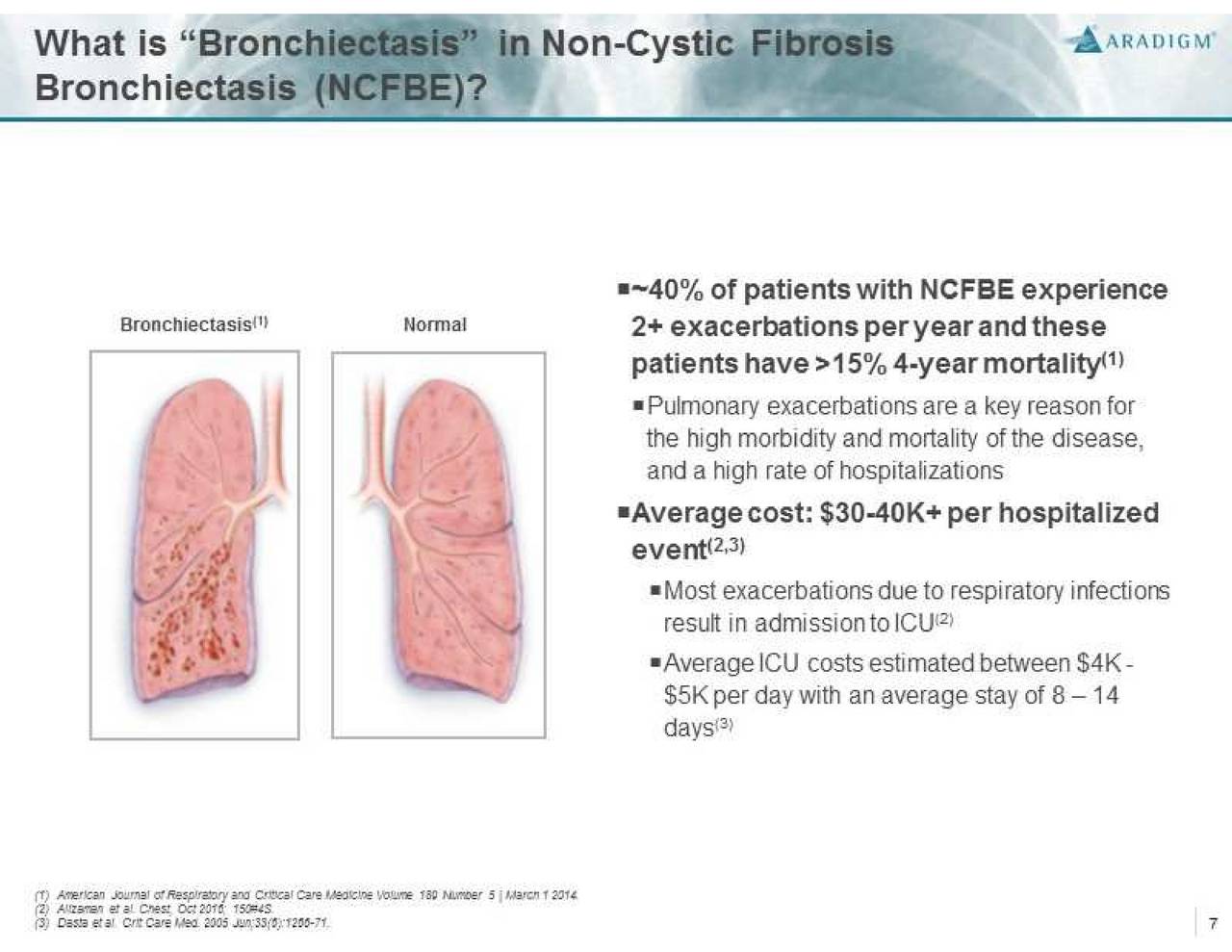
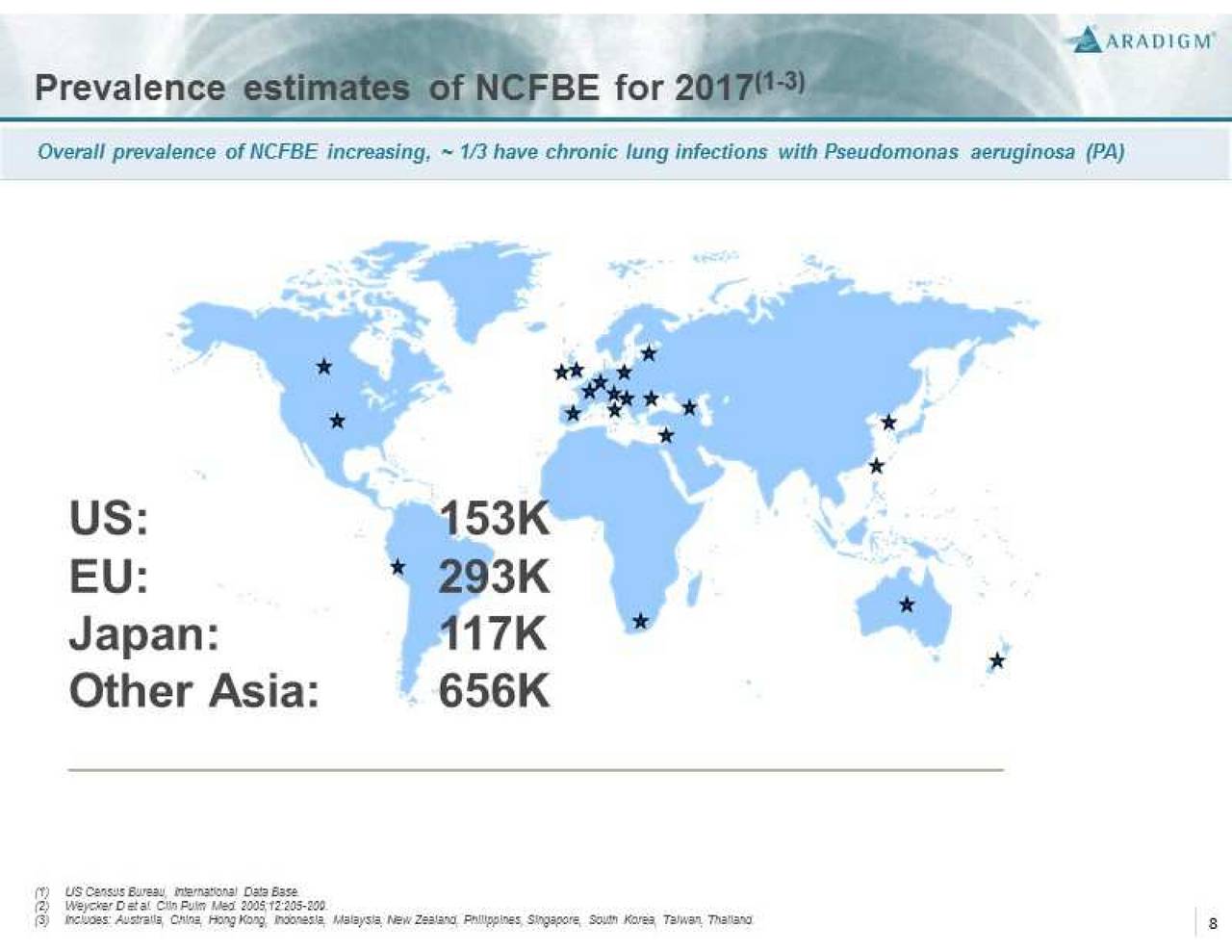
Non-Cystic Fibrosis Bronchiectasis (NCFBE) is a severe, chronic and rare disease characterized by abnormal dilatation of the bronchi and bronchioles, frequently associated with chronic lung infections. It is often a consequence of a vicious cycle of inflammation, recurrent lung infections, and bronchial wall damage. NCFBE represents an unmet medical need with high morbidity and mortality that affects more than 150,000 people in the U.S. and over 200,000 people in Europe. There is currently no drug approved for the treatment of this condition.
Forward-Looking Statements
Except for the historical information contained herein, this news release contains forward-looking statements that involve risk and uncertainties, including the risk that Apulmiq/Linhaliq may not receive regulatory approval or be successfully commercialized, as well as the other risks detailed from time to time in the Company’s filings with the Securities Exchange Commission (SEC), including the Company’s Annual Report on Form 10-K for the year ended December 31, 2017 filed with the SEC on March 23, 2018, and the Company’s Quarterly Reports on Form 10-Q.
More information about Aradigm can be found at www.aradigm.com.
Aradigm and the Aradigm Logo are registered trademarks of Aradigm Corporation. Apulmiq and Linhaliq are a registered trademark of Grifols, S.A.
ARDM changed to ARDMQ:
https://otce.finra.org/otce/dailyList?viewType=Symbol%2FName%20Changes
ARDM delisted from the Nasdaq to the OTC:
https://otce.finra.org/otce/dailyList?viewType=Additions
Nice volume spike and pop in price!
I got buy order in for Monday at $1.57, looks cheap here versus potential.
There was no "FDA denial" when you wrote this post. Maybe you confused that with the FDA's requirement of a name change to Linhaliq.
Boxer Capital Llc Reports 5.9 Percent Passive Stake In Aradigm Corp As Of November 30 - Sec Filing
https://www.sec.gov/Archives/edgar/data/1465837/000119312517366366/0001193125-17-366366-index.htm
FORM 4 - SEC Filing
GONDA IGOR - Stock Option (Right to Buy) 60000 shares
https://www.sec.gov/Archives/edgar/data/1013238/000112329217001763/xslF345X03/edgar.xml
PECOTA NANCY E - Stock Option (Right to Buy) 40000 shares
https://www.sec.gov/Archives/edgar/data/1013238/000112329217001762/xslF345X03/edgar.xml
Froehlich Juergen Kurt - Stock Option (Right to Buy) 50000 shares
https://www.sec.gov/Archives/edgar/data/1013238/000112329217001761/xslF345X03/edgar.xml
January 11, 2018: Antimicrobial Drugs Advisory Committee public meeting (Hyattsville, MD) - The committee will discuss new drug application (NDA) 210693, ciprofloxacin dispersion for inhalation, sponsored by Aradigm Corp., for the proposed indication of treatment of non-cystic fibrosis bronchiectasis patients with chronic lung infections with Pseudomonas aeruginosa.
Aradigm Announces FDA Advisory Committee Meeting for Linhaliq
http://investor.aradigm.com/releasedetail.cfm?ReleaseID=1050394
The FDA's Antimicrobial Drugs Advisory Committee will meet on Thursday, January 11 to discuss Aradigm's New Drug Application (NDA) seeking approval for Linhaliq (ciprofloxacin) for the treatment of non-cystic fibrosis bronchiectasis in patients with chronic infections due to Pseudomonas aeruginosa.
35Mil AS/15Mil OS <= Nice!
https://www.sec.gov/Archives/edgar/data/1013238/000119312517333059/R3.htm
XML 15 R3.htm IDEA: XBRL DOCUMENT
Condensed Consolidated Balance Sheets (Parenthetical) - $ / shares
Sep. 30, 2017
Statement of Financial Position [Abstract]
Preferred stock, shares authorized
5,000,000
Preferred stock, shares outstanding
0
Common stock, par value
Common stock, shares authorized
35,045,765
Common stock, shares issued
14,951,089
Common stock, shares outstanding
14,951,089
Aradigm (ARDM) Shares March Higher, Can It Continue?
https://www.zacks.com/stock/news/278799/aradigm-ardm-shares-march-higher-can-it-continue
Man I gave up on this stock when we received the FDA denial. Never sold though. I remember buying my first shares at .12 before the reverse split. Friend pinged me the other day and said hey what's going on with ardm. I had not looked at my ardm account in 6 months. I was like WTF. I am feeling good that this time is our time. Wow over 5 years holding this stock. Good luck true longs that are still around.
Aradigm Regains Compliance with NASDAQ Listing Requirements
http://investor.aradigm.com/releasedetail.cfm?ReleaseID=1043285
Key financials in the licensing ageement:
Partnership with Grifols, S.A.
* The royalty rate is 12.5% on the first $300 milion of annual sales for all territories and all indications, and 20% for annual sales in excess of $300 milion
* $20 milion in pre-approval and approval milestones remaining
* Aradigm responsible for the product supply
Aradigm believes the NCFBE indication for Linhaliq exceed $500MM of sales by 2021. Ladenburhg analysts project $1B sales by 2022, Nektar projected $750 milion for their NCFBE product with Bayer.
Key Investment Highlights
* Aradigm believes the NCFBE indication for Linhaliq exceed $500MM of sales by 2021.
Ladenburhg analysts project $1B sales by 2022, Nektar projected $750 milion for their NCFBE product with Bayer
* US Orphan Drug Designation with no existing approved therapies
* QIDP Designation and Fast Track received
FDA priority review accompanies QIDP designation
* Strong IP protection to 2031
8 patents issued in US; 41 issued patents in 37 countries outside the US
* Important objectives achieved in Phase 3
Prevention and reduction in frequency of pulmonary exacerbations
High persistent drug contrentrations in sputum, low in blood
Sustained powerful antipseudomonal activity even for patients with resistant isolates
Good safety and tolerability
* Commercial Launch in US expected in Q2'2018
EU approval expected Q4'18/Q1'19
* Sole competitor: Bayer's twice dily, dry powder inhaled ciprofloxacin
* Linhaliq is administered once daily:
Active in biofilms, uptake by macrophages
The company has a lot of potential for future growth.
New vid chart? ![]()
Market value estimate.
See https://seekingalpha.com/article/4108783-aradigm-hidden-binary-event-russian-roulette-fda-spinning-chamber
The part of the page 20:
I made some quick calculations to try to estimate Aradigm's market value.
Grifols will fully commercialize Linhaliq in exchange for between 12.5% and 20% of revenues, by country, with revenues divided by half if there's a competitor in that country. I expect Bayer to begin to become a competitor at least six months after Aradigm and potentially longer if it needs to do another trial, so I estimate 12% royalties. Given that the royalties from revenues are pure earnings, I assign a 12 P/E from the revenue stream. I assume a 15% discount rate, 2021 sales of $750m, and $20m more in milestone payments. I discount the rest of the pipeline to zero.
Given:
A 15% discount rate,
2021 sales of $750m,
12% royalties and a 12 P/E,
a cumulative 40% dilution, and
$20m more in milestone payments...
...then working backwards, Aradigm should be worth perhaps (at least) ~$420m now (the discount rate here factoring in all risk, including chances), which is 18.7 times the market cap as of 9/21/2017 of ~$22.67m...
Aradigm (ARDM) PT Raised to $10 at Ladenburg Thalmann
Ladenburg Thalmann analyst Matthew Kaplan raised his price target on Aradigm (NASDAQ: ARDM) to $10.00 (from $7.50) while maintaining a Buy rating as FDA accepts Linhaliq NDA with priority review.
The analyst notes the PDUDA date is 1/26/18.
Still Running like a beast lol
ARDM monster back in
Damn nice move! Should have held a bit longer but can't watch too many plays at once.
Yeah was great, I exited yesterday from last weeks loads
Just Beautyful
* * $ARDM Video Chart 09-25-17 * *
Link to Video - click here to watch the technical chart video
FDA news!! Boom!
* * $ARDM Video Chart 09-22-17 * *
Link to Video - click here to watch the technical chart video
Moving up on no Volume
No more shares for shorty left:)
Did. Just Grab some more around 2,35
I'll grab more before close for next week announcement .
I belive so too
I bought that 1,20 quiet a while back and let it sit. Now this Could b 3bagger plus for me i guess
Thinking about buying more here around 2,60
Float higher than 800k but still tiny, shorts piling on, perfect short trap being setup.
800k can be devoured easily, this is gonna be a beast.
This is Looking mighty good
FDA news should be coming next week, 60 days is on 26th
|
Followers
|
41
|
Posters
|
|
|
Posts (Today)
|
0
|
Posts (Total)
|
1434
|
|
Created
|
11/23/04
|
Type
|
Free
|
| Moderators | |||




NEW YORK, NY--(Marketwired - Oct 29, 2013)
"Aradigm's Lipoquin and Pulmaquin inhalable antibiotics are potential best-in-class treatments designed for chronic use and to be effective at treating bacterial infections in patients' lungs, while also minimizing lung irritation that is common in cystic fibrosis and especially bronchiectasis patients," said Andrew I. McDonald, Ph.D., Founding Partner at LifeSci Advisors. "The recent partnership with Grifols provides adequate funding to bring the program through Phase III trials in bronchiectasis and may allow Aradigm to pursue additional indications."
http://www.marketwired.com/press-release/lifesci-advisors-initiates-coverage-of-aradigm-corporation-otcbb-ardm-1845844.htm
Phase III Program in Bronchiectasis Planned to Launch in 2014; Recent Partnership With Grifols Worth Up to $90 Million Plus Tiered Royalties;
ALBANY, New York, February 25, 2014 /PRNewswire/
Pulmonary Drug Delivery Systems Market Expected to Reach USD 28.7 Billion Globally in 2019: Transparency Market Research
According to a new market report published by Transparency Market Research "Pulmonary Drug Delivery Systems Market(Products- Metered Dose Inhalers, Dry Powder Inhalers and Nebulizers; Applications- Asthma, COPD and Cystic Fibrosis) - Global Industry Analysis, Size, Share, Growth, Trends and Forecast, 2013 - 2019," the global pulmonary drug delivery systems market was valued at USD 21.03billion in 2012 and is expected to grow at a CAGR of4.5% from 2013 to 2019, to reach an estimated value of USD 28.70billion in 2019.
http://www.prnewswire.com/news-releases/pulmonary-drug-delivery-systems-market-expected-to-reach-usd-287-billion-globally-in-2019-transparency-market-research-247046411.html
HYATTSVILLE, Maryland Tue Apr 1, 2014 7:49pm EDT
U.S. FDA advisers back MannKind's inhaled diabetes drug Afrezza
http://www.reuters.com/article/2014/04/01/us-mannkind-fda-diabetes-idUSBREA301Z620140401
ARDM has huge potential with AERx iDMS (inhalable insulin) right now.
The drug was in the last lap of clinical trials before halting by Novo Nordisk in past.
http://www.aradigm.com/products_iDMS.html
http://www.aradigm.com/products_1700.html
https://www.visiongain.com/Report/1252/Drug-Delivery-Technologies-Market-Forecast-2014-2024
Sept 22, 2017
Aradigm to Present at the Ladenburg Thalmann 2017 Healthcare Conference on September 26
http://investor.aradigm.com/releasedetail.cfm?ReleaseID=1041356
https://seekingalpha.com/article/4109924-aradigm-corporation-ardm-presents-ladenburg-thalmann-2017-healthcare-conference-slideshow
Sept 27, 2017
Aradigm Announces FDA Acceptance of NDA for Linhaliq with Priority Review Status
http://investor.aradigm.com/releasedetail.cfm?ReleaseID=1041475
Oct 10, 2017
Aradigm Regains Compliance with NASDAQ Listing Requirements
http://investor.aradigm.com/releasedetail.cfm?ReleaseID=1043285
SEC Company Filings - Aradigm Corporation
https://searchwww.sec.gov/EDGARFSClient/jsp/EDGAR_MainAccess.jsp?search_text=*&sort=Date&formType=1&isAdv=true&stemming=true&numResults=10&queryCo=Aradigm&numResults=10
| Volume | |
| Day Range: | |
| Bid Price | |
| Ask Price | |
| Last Trade Time: |
